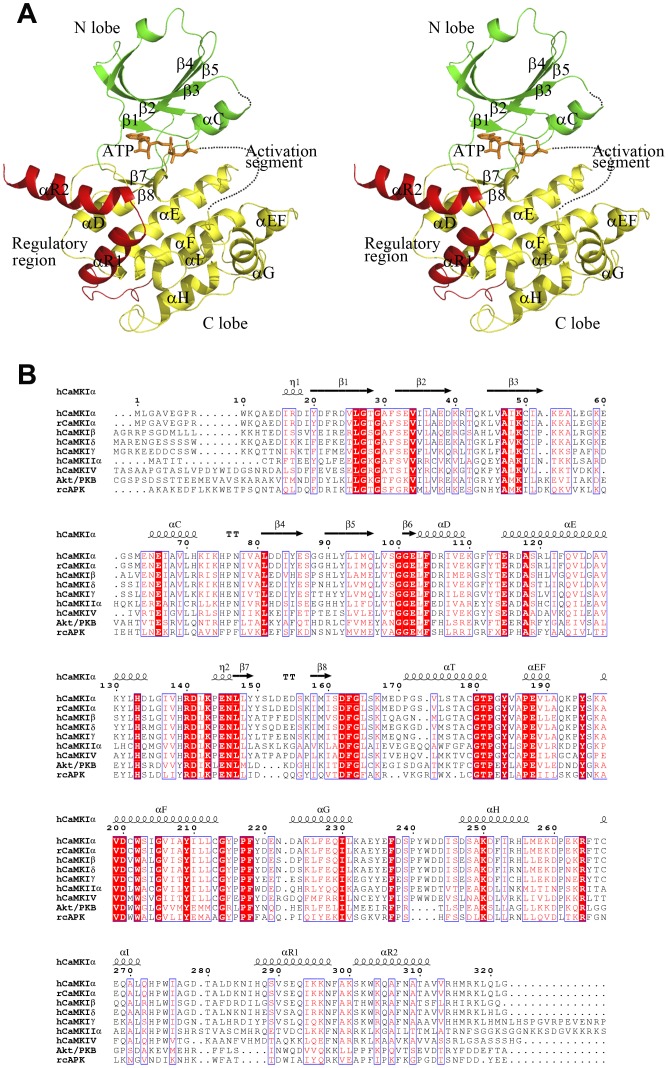
Figure 1. Structure of CaMKI.
A A stereoview of the overall structure of CaMKI320-ATP. The various CaMKI truncation forms have similar overall structure and the CaMKI320-ATP complex is selected as a representative. CaMKI consists of a catalytic core containing the N lobe (green), the C lobe (yellow) and a regulatory region (red). The bound ATP is shown with a ball-and-stick model (orange). The activation segment is primarily disordered and denoted with a dashed curve in orange. B Sequence alignment of human CaMKI (isoforms α, β, δ, and γ), rat CaMKIα, human CaMKIIα, human CaMKIV, human Akt/PKB, and rat cAPK. The highly conserved residues are indicated with closed red boxes, and the conserved residues with open red boxes. The secondary structure elements of human CaMKIα are indicated on the top of the alignment.