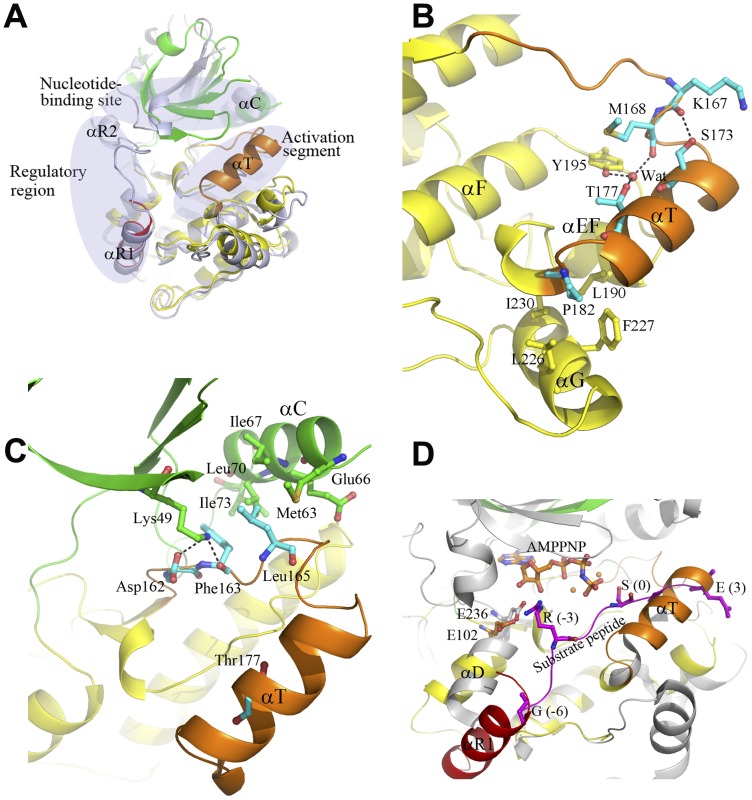
Figure 2. A distinctive autoinhibited state of CaMKI.
A Comparison of the apo human CaMKI320 and the apo rat CaMKI320 (PDB code 1A06) [16]. The apo human CaMKI320 is colored as in Figure 1A. The apo rat CaMKI320 is colored in light blue. Substantial conformational differences are observed between the two structures in the regulatory region, the activation segment, and the nucleotide-binding site which are indicated with ovals. In particular, the C-terminal part of the activation segment encompassing residues Pro171 to Thr181 assumes a rare helical conformation (helix αT). B Hydrophilic and hydrophobic interactions of helixαT with the surrounding residues in the apo CaMKI320. The involved residues are shown with ball-and-stick models, and the hydrogen-bonding interactions are indicated with dashed lines. C Interactions of the N-terminal part of the activation segment with the N lobe in the apo CaMKI320. The N-terminal part of the activation segment including the DFG motif and the following Leu165 form both hydrophilic and hydrophobic interactions with helix αC and strand β3 of the N lobe. The involved residues and additionally Glu66 on helix αC are shown with ball-and-stick models, and the hydrogen-bonding interactions are indicated with dashed lines. D Comparison of the apo CaMKI320 and the Akt/PKB-GSK3β complex in the substrate-binding site. In the Akt/PKB-GSK3β complex (gray), the bound GSK3β peptide is shown with a magenta ribbon, and the bound AMPPNP is shown with a ball-and-stick model in orange. Residues at P(-6), P(-3), P0, and P+3 positions of the GSK3β peptide, Glu236 of Akt/PKB which forms a salt bridge with Arg at P(-3) of the peptide, and the equivalent Glu102 in the apo CaMKI320 are shown with ball-and-stick models.