Abstract
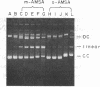
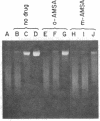
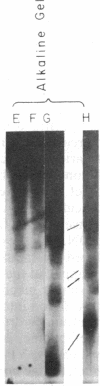
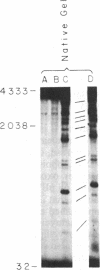
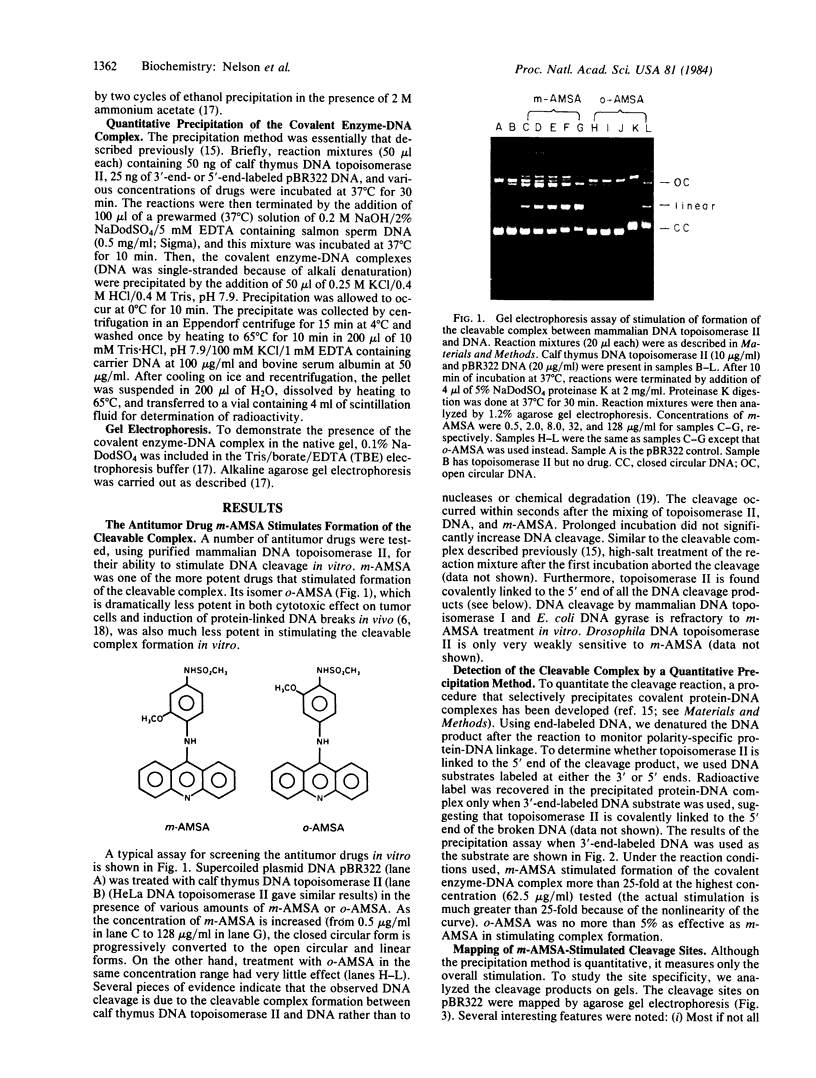
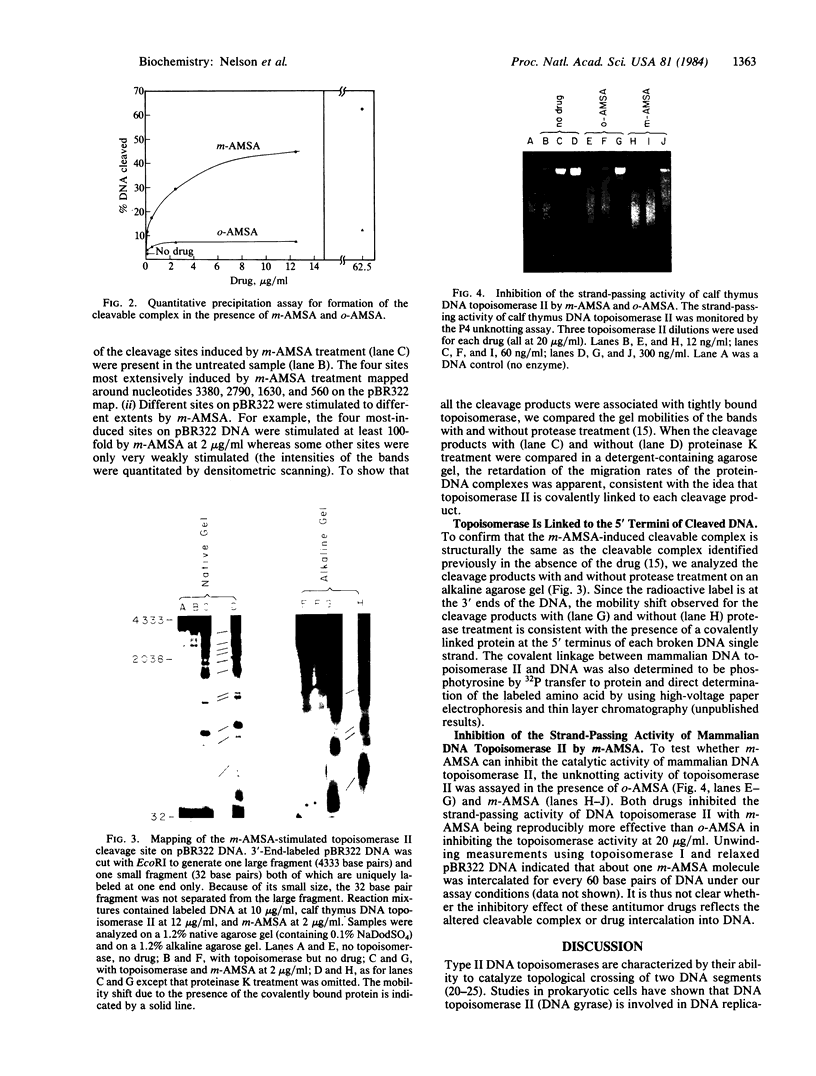
The intercalative acridine derivative 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA), but not its isomer o-AMSA, is a potent antitumor drug that in mammalian cells stimulates the formation of DNA strand breaks that are characterized by tightly bound proteins. Using purified mammalian DNA topoisomerases, we have analyzed the effects of these antitumor drugs on topoisomerase-DNA interactions. The antitumor drug m-AMSA dramatically stimulates the formation of a topoisomerase II-DNA complex that is detected on protein-denaturant treatment: both single- and double-stranded DNA breaks are produced and a topoisomerase II subunit is linked covalently to each 5' end of the broken DNA strands. The noncytotoxic isomer, o-AMSA, which does not induce significant amounts of DNA breaks in cultured cells, exhibits a correspondingly smaller effect in stimulating formation of the complex in vitro. The agreement between in vitro and in vivo studies suggests that mammalian DNA topoisomerase II may be the primary target of m-AMSA and that the drug-induced complex formation between topoisomerase II and DNA may be the cause of cytotoxicity and other effects such as DNA sequence rearrangements and sister-chromatid exchange.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain B. F., Atwell G. J. The experimental antitumour properties of three congeners of the acridylmethanesulphonanilide (AMSA) series. Eur J Cancer. 1974 Aug;10(8):539–549. doi: 10.1016/0014-2964(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Oka M. S., Tobey R. A. Cell-cycle-specific chromosome damage following treatment of cultured Chinese hamster cells with 4'-[(9-acridinyl)-amino]methanesulphon-m-anisidide-HCl. J Natl Cancer Inst. 1978 May;60(5):1155–1161. [PubMed] [Google Scholar]
- Drewinko B., Yang L. Y., Barlogie B. Lethal activity and kinetic response of cultured human cells to 4'-(9-acridinylamino)methanesulfon-m-anisidine. Cancer Res. 1982 Jan;42(1):107–111. [PubMed] [Google Scholar]
- Furlong N. B., Sato J., Brown T., Chavez F., Hurlbert R. B. Induction of limited DNA damage by the antitumor agent Cain's acridine. Cancer Res. 1978 May;38(5):1329–1335. [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Cozzarelli N. R. The binding of gyrase to DNA: analysis by retention by nitrocellulose filters. Nucleic Acids Res. 1982 Nov 11;10(21):6833–6847. doi: 10.1093/nar/10.21.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Davis J. L., Calendar R. Novel topologically knotted DNA from bacteriophage P4 capsids: studies with DNA topoisomerases. Nucleic Acids Res. 1981 Aug 25;9(16):3979–3989. doi: 10.1093/nar/9.16.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Majumdar K. C., Chang R. Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem Biophys Res Commun. 1977 Jun 6;76(3):705–710. doi: 10.1016/0006-291x(77)91557-1. [DOI] [PubMed] [Google Scholar]
- Marshall B., Ralph R. K., Hancock R. Blocked 5'-termini in the fragments of chromosomal DNA produced in cells exposed to the antitumor drug 4'-[(9-acridinyl)-amino]methanesulphon-m-anisidide (mAMSA). Nucleic Acids Res. 1983 Jun 25;11(12):4251–4256. doi: 10.1093/nar/11.12.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Paoletti C., Lesca C., Cros S., Malvy C., Auclair C. Ellipticine and derivatives induce breakage of L1210 cells DNA in vitro. Biochem Pharmacol. 1979;28(3):345–350. doi: 10.1016/0006-2952(79)90096-0. [DOI] [PubMed] [Google Scholar]
- Ralph R. K. On the mechanism of action of 4'-[(9-acridinyl)-amino] methanesulphon-m-anisidide. Eur J Cancer. 1980 May;16(5):595–600. doi: 10.1016/0014-2964(80)90198-x. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Bradley M. O. DNA double-stranded breaks in mammalian cells after exposure to intercalating agents. Biochim Biophys Acta. 1981 Jun 26;654(1):129–134. doi: 10.1016/0005-2787(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D. L., Kohn K. W. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978 Jun 22;519(1):23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Sander M., Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8421–8428. [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Baguley B. C., Wakelin L. P., Waring M. J. Interaction of the antitumor drug 4'-(9-acridinylamino)methanesulfon-m-anisidide and related acridines with nucleic acids. Mol Pharmacol. 1981 Sep;20(2):404–414. [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Michaels S. Cytotoxicity and DNA strand breaks by 5-iminodaunorubicin in mouse leukemia L1210 cells: comparison with adriamycin and 4'-(9-acridinylamino)methanesulfon-m-anisidide. Cancer Res. 1982 Jul;42(7):2687–2691. [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Kerrigan D., Pommier Y., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks produced in mouse leukemia L1210 cells by ellipticine and 2-methyl-9-hydroxyellipticinium. Biochem Pharmacol. 1982 Oct 15;31(20):3261–3267. doi: 10.1016/0006-2952(82)90560-3. [DOI] [PubMed] [Google Scholar]