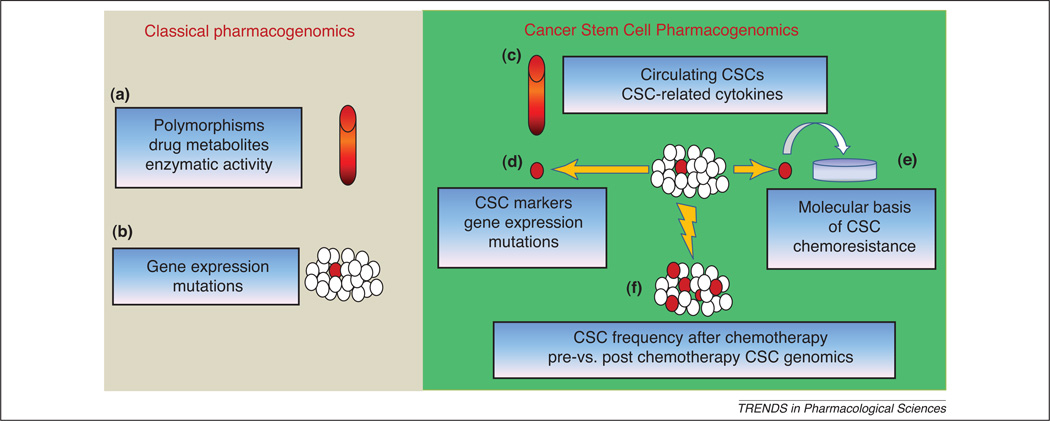
Figure 3. Design of innovative pharmacogenomic studies.
Classical pharmacogenomics deals with analysis performed on blood samples (a) or tumor specimens (b). In the first case, germinal polymorphisms or drug metabolism are studied to predict chemotherapy-related toxicity. In the second case, genomic analyses are performed on a mixed population, generally comprising a small percentage of CSCs (in red). CSC-pharmacogenomics should include detection of circulating CSCs and measurement of CSC-related cytokines from blood samples (c). In addition, CSCs isolated from primary tumors (d) are suitable for specific genomic and proteomic analysis. CSCs can also be expanded in vitro in order to perform drug sensitivity tests (e). Finally, if CSCs are more resistant, chemotherapy may increase their percentage within the tumor. Thus, analysis of CSC frequency after neoadjuvant chemotherapy, as well as comparison of pre- vs post- chemotherapy CSC genomics could predict patient response to treatment (f).