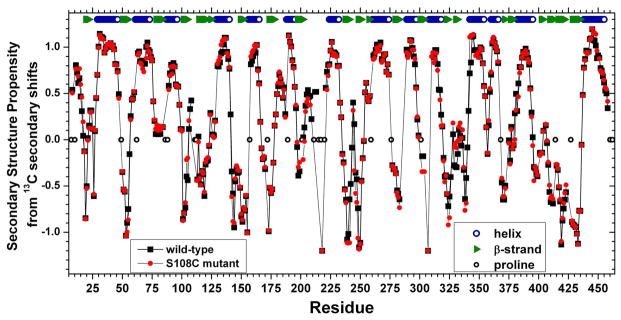
Figure 3.
Secondary structure propensities (SSP) from 13Cα and 13Cβ secondary chemical shifts. SSP values are indicated for wt enzyme (black squares) and S108C-substituted enzyme (red circles). The values were calculated over a sliding window of five residues, avoiding glycine and each residue prior to proline.29 13C referencing was to DSS. The locations of secondary structure in crystal structures16 is marked at top for comparison with SSP trends in solution.