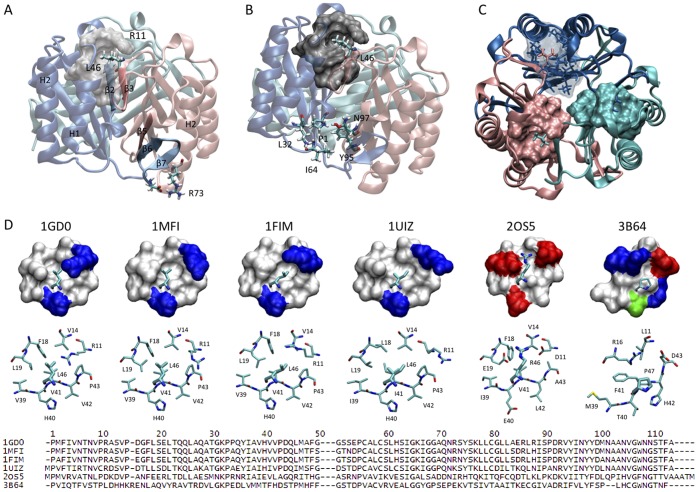
Figure 1. The Leu46 hydrophobic pocket is highly conserved across MIF species.
The three MIF monomers are represented as cartoons and are colored in pink, cyan and blue. (A) Side view of the trimer illustrating the different intersubunit interactions. Two main regions within each monomer were shown to be responsible for the protein trimerization: each subunit interacts with one neighbouring monomer through tight interactions involving the inner β-strand (β3) and with the other neighbouring monomer though the C-terminal β-hairpin (β6 and β7). The C-terminal β-hairpin comprises two major types of interactions: 1) intersubunit β-sheet, and 2) salt-bridge interactions. The β-strand β3 contributes to trimer stabilization through two types of contacts: 1) intersubunit β-sheet formation, and 2) hydrophobic interactions between the side chain of Leu46 localized on the β-strand β3 and a hydrophobic pocket from the adjacent monomer constituted by residues Arg11, Val14, Phe18, Leu19, Val39, His40, Val41, Val42, Pro43. (B) Side view of the trimer illustrating the distance between the Leu46 hydrophobic pocket and the enzymatic site. (C) Top view of huMIF showing the three hydrophobic pockets where Leu46 from adjacent monomers are packed. Each pocket is represented with the same color as the subunit it belongs to, Leu46 are represented as stick models licorice and colored with the same subunit color. Structural data according to Orita et al. [51], PDB code: 1GD0. (D) Hydrophobic pocket structure homology between different MIF species. Structural data were generated using the following PDB files: 1GD0 (human MIF, [51]); 1MFI (mouse MIF, [68]); 1FIM (rat MIF, [69]); 2OS5 (Ancylostoma ceylanicum MIF, [70]); 3B64 (Leishmania parasite MIF, [71]); 1UIZ (Xenopus laevis MIF, [72]). Amino acids are represented on the figure with one-letter codes.