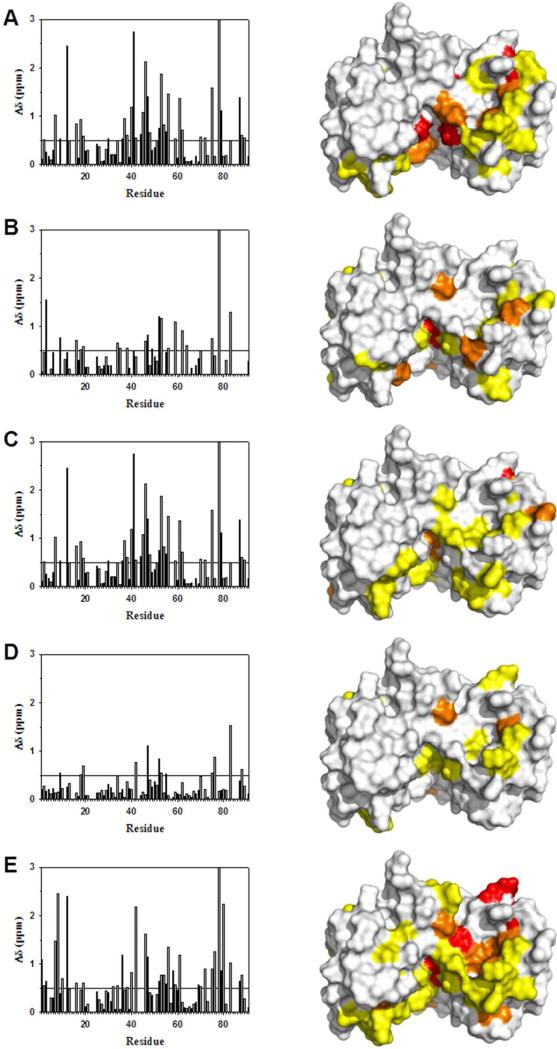
Figure 3.
Changes in backbone 1H and 15N chemical shifts for Ca2+-bound S100B upon binding various peptide targets. Similar residues in S100B undergo chemical shift perturbations upon binding TRTK12(265-276) (Panel A), p53(367–388) (Panel B), NDR(62-87) (Panel C), HDM2(25-47) (Panel D), or HDM4(25-47) (Panel E) peptides. The bar graphs show the combined backbone 1H and 15N chemical shift perturbations upon peptide binding to Ca2+–S100B. Residues without Δδ values underwent exchange broadening upon binding, and/or could not be assigned in both the peptide-free and peptide bound states.