Figure 1. Effect of systemic HSP65 administration on airway responses in a secondary allergen challenge model.
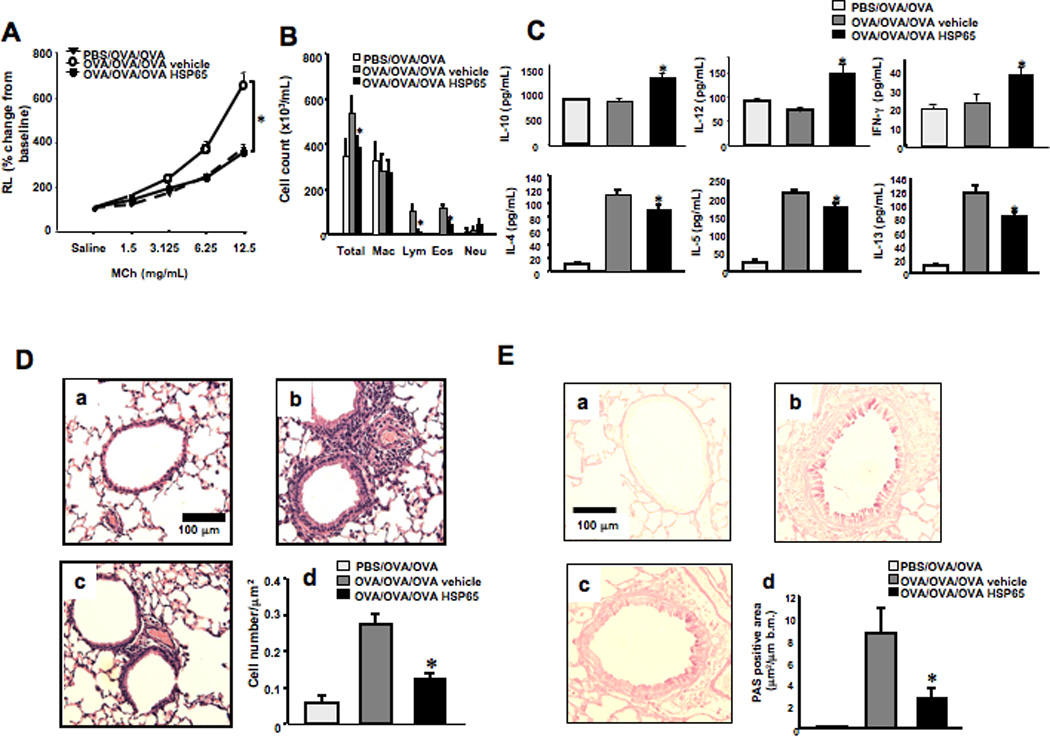
The effects of intraperitoneal injection of HSP65 were determined in a secondary allergen challenge model. Control groups received vehicle. (A) Lung resistance changes in response to increasing doses of MCh, expressed as a percent of baseline (saline) values. (B) Cell composition in BAL fluid. Macro; macrophages, Lympho; lymphocytes, Eos; eosinophils, Neu; neutrophils. (C) BAL fluid cytokine levels. (D) Lung tissue histology (H&E) and quantitation of inflammatory cells. (E) Periodic acid-Schiff (PAS) staining and quantitation of goblet cells. Panel a: Sham sensitization and primary and secondary OVA challenge, Panel b: secondary challenge following vehicle treatment, and panel c: secondary challenge following HSP65 treatment. Mice were sham sensitized followed by primary and secondary OVA challenge (PBS/OVA/OVA) or OVA sensitized followed by primary and secondary OVA challenge (OVA/OVA/OVA). (n=8). *p<0.05 compared to OVA/OVA/OVA vehicle.
