Figure 2.
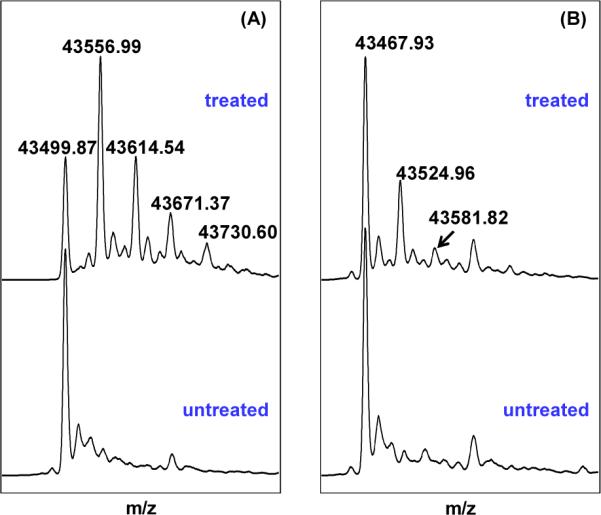
(A) Deconvoluted ESI-MS spectrum of the WT SPL enzyme treated by excess iodoacetamide under native conditions. Comparing with the untreated protein, the major species in treated enzyme exhibits a mass gain of 57.13. The intensity of this peak is much stronger than those corresponding to proteins which carry 2, 3 or 4 labels. This observation suggests that one of the four cysteines in WT SPL is prone to alkylation. (B) Deconvoluted ESI-MS spectrum of the SPL C141A mutant treated by excess iodoacetamide under an identical condition. The mono-labeled species in the treated C141A mutant exhibited a peak whose intensity is comparable with that of the treated WT enzyme carrying two alkyl labels, but much weaker than that corresponding to the mono-alkylated WT enzyme, suggesting that the alkylation site is at one of the three cluster cysteines. The different behavior toward the iodoacetamide treatment between these two proteins suggests that C141 residue in the WT SPL is accessible from the aqueous solution.
