Figure 1.
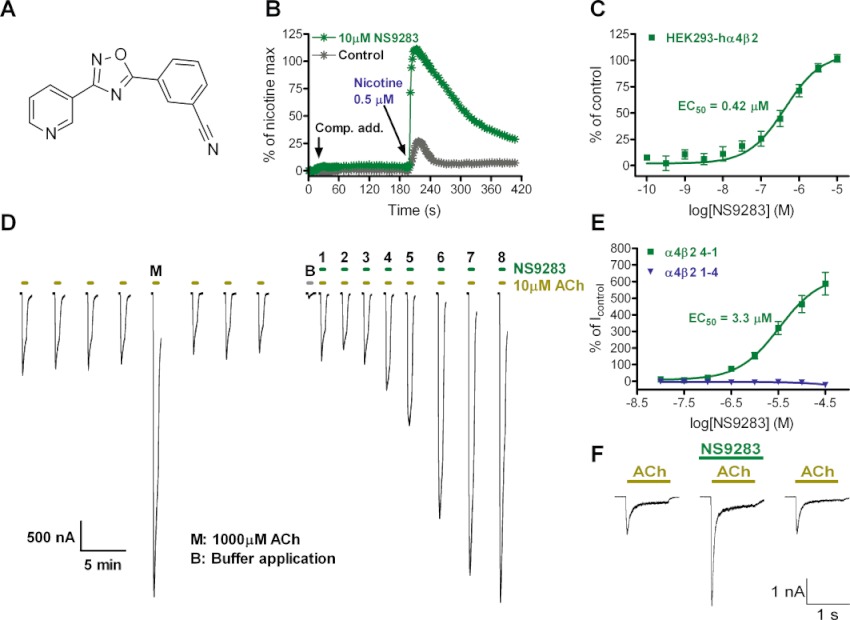
Allosteric modulation of α4β2 nACh receptors by NS9283. (A) Chemical structure of 3-[3-(3-pyridyl)-1,2,4-oxadiazol-5-yl]benzonitrile (NS9283). (B) NS9283 fails to activate α4β2 nACh receptors but increases the amplitude of nicotine-evoked Ca2+-responses. NS9283 or control buffer was added to HEK293-hα4β2 cells after a 20 s stabilization period, and a ∼EC20 concentration of nicotine was added after 200 s. Response amplitudes were background subtracted and normalized to the effect of a saturating nicotine concentration (100 µM). The traces shown represent average responses from 16 wells for each experiment. (C) Concentration–response relationship for NS9283 allosteric modulation. Nicotine-induced (0.5 µM) Ca2+-responses (baseline subtracted and normalized; presented as mean ± SEM of four experiments) were fitted to the Hill equation by non-linear regression. (D) Representative current traces of ACh and NS9283+ACh in two-electrode voltage-clamp experiments in Xenopus laevis oocytes. The oocyte was injected with α4 and β2 nACh receptor subunits in a 4:1 ratio. Application of ACh or NS9283 mixed with ACh is indicated by a bar above each trace. NS9283 concentrations were increased in half log unit increments starting with lowest concentration (10 nM) in application ‘1’ and ending with highest concentration (31.6 µM) in application ‘8’. (E) Concentration–response relationships for NS9283 modulation of ACh-evoked currents in X. laevis oocytes. Oocytes were injected with α4 and β2 nACh receptor subunits in a 1:4 or 4:1 ratio to obtain (α4)2(β2)3 or (α4)3(β2)2 receptors, respectively. Peak current amplitudes were baseline subtracted and normalized with respect to the current induced by ACh (1 µM for (α4)2(β2)3, 10 µM for (α4)3(β2)2), and are presented as mean ± SEM of 11–12 experiments. Data points for (α4)3(β2)2 were fitted to the Hill equation by non-linear regression, and the result is seen in Table 1. (F) Representative current traces from whole-cell patch-clamp experiments on human α4β2 nACh receptors transiently expressed in GH4C1 cells. Cells were clamped at −60 mV and stimulated with ACh (100 µM) in the absence, presence and after wash-out of NS9283 (10 µM), respectively.
