Abstract
Background
Abdominal aortic aneurysm (AAA) formation is characterized by inflammation, smooth muscle activation and matrix degradation. This study tests the hypothesis that CD4+ T cell-produced IL-17 modulates inflammation and smooth muscle cell activation leading to the pathogenesis of AAA and that human mesenchymal stem cell (MSC) treatment can attenuate IL-17 production and AAA formation.
Methods and Results
Human aortic tissue demonstrated a significant increase in IL-17 and IL-23 expression in AAA patients compared to controls as analyzed by RT-PCR and ELISA. AAA formation was assessed in C57BL/6 (wild type; WT), IL-23−/− or IL-17−/− mice using an elastase-perfusion model. Heat-inactivated elastase was used as control. On days 3, 7 and 14 following perfusion, abdominal aorta diameter was measured by video micrometry, and aortic tissue was analyzed for cytokines, cell counts and IL-17-producing CD4+ T cells. Aortic diameter and cytokine production (MCP-1, RANTES, KC, TNF-α, MIP-1α and IFN-γ) was significantly attenuated in elastase-perfused IL-17−/− and IL-23−/− mice compared to WT mice on day 14. Cellular infiltration (especially IL-17-producing CD4+ T cells) was significantly attenuated in elastase-perfused IL-17−/− mice compared to WT mice on day 14. Primary aortic smooth muscle cells were significantly activated by elastase or IL-17 treatment. Furthermore, MSC treatment significantly attenuated AAA formation and IL-17 production in elastase-perfused WT mice.
Conclusion
These results demonstrate that CD4+ T cell-produced IL-17 plays a critical role in promoting inflammation during AAA formation and that immunomodulation of IL-17 by MSCs can offer protection against AAA formation.
Keywords: abdominal aortic aneurysms, interleukins, inflammation, stem cells, lymphocytes
Abdominal aortic aneurysm (AAA) formation involves chronic inflammation, upregulation of proteolytic pathways, oxidative stress and loss of arterial wall matrix.1, 2 The critical inflammatory pathways that lead to smooth muscle cell activation and apoptosis during AAA formation remain to be elucidated. Previous studies have postulated an important role for pro-inflammatory cytokines in various mouse models of AAA.3–5 However, the specific role of cytokines in the modulation of inflammation, oxidative stress, smooth muscle apoptosis and vascular remodeling remains to be defined. IL-17, a key T cell- produced pro-inflammatory cytokine, is known to be an important regulator of inflammation and apoptosis in chronic inflammation.6, 7 Previous studies have also demonstrated a predominance of CD4+ T cells in AAA.8, 9 However, the contribution, cellular sources and signaling pathways of CD4+ T cells and IL-17 remain unknown. Thus, the present study investigates the hypothesis that CD4+ T cell-produced IL-17 modulates inflammation and smooth muscle cell activation in the pathogenesis of AAA. Human aortic tissue from AAA patients, a murine elastase-perfusion AAA model and in vitro studies were utilized to test this hypothesis.
Recent studies have raised the possibility of stem cell therapies for improving the outcome of inflammation-based diseases including aortic aneurysms.10–12 Mesenchymal stem cells (MSCs) are multipotent with the capability to differentiate into a wide range of cell types.13 Another fundamental property of MSCs is the immunosuppressive activities which are postulated to have tremendous potential to translate to novel therapeutic strategies for tissue repair and immunomodulation.14, 15 Therefore, in the pursuit of pharmacological modalities for AAA, the immunomodulatory effects of MSCs on the pathogenesis of AAA was investigated in the murine elastase-perfusion AAA model.
Methods
Human Aortic Tissue Analysis
Collection of human aortic tissue was approved by the University of Virginia’s Institutional Review Board (protocol number 13178). Preoperative consent was obtained from all patients. AAA tissue from male patients was resected during open surgical AAA repair, and abdominal aortic tissue was obtained from transplant donor patients to serve as controls. Tissue was homogenized in Trizol, and RNA was purified per manufacturer’s protocol (Qiagen, Valencia, CA). cDNA was synthesized using iScript™ cDNA Synthesis Kit (BioRad, Hercules, CA). Quantitative (real-time) RT-PCR was performed with primer sets (MWG/Operon, Huntsville, AL) in conjunction with SsoFast™ EvaGreen® Supermix (BioRad, Hercules, CA). Primers used were as follows: GAPDH forward, CATTGTGGAAGGGCTCATGA; GAPDH reverse, TCTTCTGGGT GGCAGTGATG; IL-23p19 forward, GAGCAGCAACCCTGAGTCCCTA; IL-23p19 reverse, CAAATTTCCCTTCCCATCTAATAA; IL-17 forward, ATGACTCCTGGGAAGACC TCATTG; IL-17 reverse, TTAGGCCACATGGTGGACAATCGG. Gene expression was calculated by using the relative quantification method according to the following equation: 2(−ΔCT), where ΔCT = (Average gene of interest) − (Average reference gene), where GAPDH was used as the reference gene.
Animals
All animal experimentation was approved by the University of Virginia’s Institutional Animal Care and Use Committee. Male C57BL/6, IL-17A−/− and IL-23−/− mice (8-12 weeks of age) were used. C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). IL-17A−/− and IL-23−/− (p19 subunit knockout) mice, which were backcrossed onto C57BL/6 background for 10 generations, were obtained from Dr. Yoichiro Iwakura (The Institute of Medical Sciences, University of Tokyo) and Genentech (San Francisco, CA), respectively.
Elastase Perfusion Model of Aneurysm Formation
A murine elastase perfusion model of AAA formation was used as previously described.16, 17 Briefly, the infrarenal abdominal aorta was isolated in situ and perfused with porcine pancreatic elastase (Sigma, 0.4 U/mL) for 5 minutes at a pressure of 100 mm Hg. Control animals were perfused with heat-inactivated elastase for 5 minutes. Video micrometric measurements of aortic diameters were made in situ before perfusion, after perfusion, and before harvesting the aorta on separate independent groups of mice on days 3, 7 and 14.
Enzyme-Linked Immunosorbent Spot Assay
Primary CD4+ T cells were purified from mouse aortic tissue using a magnetic bead-based cell isolation kit (Miltenyi Biotec, Germany). An IL-17A enzyme-linked immunosorbent spot (ELISPOT) assay (R&D Systems, Minneapolis, MN) was utilized as instructed by the manufacturer. Spot forming cells were counted under a microscope. Results are presented as the average number of spot-forming cells per total number of cells plated.
Cytokine Measurements
Cytokine content in aortic tissue (human and mice) homogenates was quantified using the Bioplex Bead Array technique using a multiplex cytokine panel assay (Bio-Rad Laboratories, Hercules, CA).
Purification of Primary Aortic Smooth Muscle Cells
Primary aortic smooth muscle cells were purified from C57BL/6 mice as previously described.18
Flow Cytometry
Aortic tissue from mice was minced and incubated for 15 min at 37°C with collagenase type IA (Sigma) in PBS with 0.5% BSA and 2mM EDTA. The cell suspension was prepared for flow cytometry analysis for cell counts using Caltag Counting Beads (Invitrogen), as previously described.19 Cells were blocked with anti-mouse CD16/CD32 (1 µg/mL; eBioscience) before surface labeling with the following antibodies: Aqua (2 µg/mL; Invitrogen), APC–Cy7–labeled CD45 (eBioscience), FITC-labeled B220, APC-labeled CD4, Pacific blue-labeled CD8, PerCP Cy5.5-labeled CD11b, PE-labeled Ly6-G, PE-Texas Red-labeled NK1.1 and PE-Cy 7 labeled CD11c (all BD Biosciences). FACS data were analyzed using FlowJo software 8.8.
Human Placental Mesenchymal Stem Cell Isolation
Mesenchymal stem cells (MSCs) were isolated from the chorionic villi of term human placenta as previously described.20 Further characterization of MSCs done by flow cytometry confirms a pattern consistent with MSC population showing an expression of CD90, CD73, CD105, CD29 and CD44 (Michael P. Murphy, MD; unpublished data, 2011). MSCs lacked expression of CD45, CD34, CD14, CD19, and HLA-DR. Immunofluorescence staining of embryonic stem cell antigens in MSCs demonstrated the expression of Oct-4, Nanog, SSEA-3 and SSEA-4. After isolation and subsequent culture, early passage placental MSCs were plated in a 96 well-plate at a density of 15,000 cells per designated well for the in vitro assays. Also, 1×106 MSCs were injected into WT mice via tail vein injection on day 1 in the in vivo experiments.
Mixed Lymphocyte Reactions
The mononuclear cell (MNC) fraction of peripheral blood specimens from healthy volunteers was separated using Ficoll-Paque PLUS separation (GE Healthcare Bio-Sciences AB, Piscataway, NJ). In vitro experiments of mixed lymphocyte reactions using MNCs (3×105) and MSCs was performed wherein MNCs were stimulated with mouse anti-human CD3 (1 µg/mL; BD Biosciences, San Diego, CA) and mouse anti-human CD28 (0.5 µg/mL; BD Biosciences). Cell cultures were then incubated for 6 days at 37 °C/5% CO2. Eighteen hours prior to the end of the experiment, 3H-thymidine (0·5 mCi/well) was added to each of the wells. On day 7, cell proliferation was determined by measuring the incorporation of 3H-thymidine using a beta-plate reader and quantified as counts per minute (cpm). Cell culture supernatants were analyzed for IL-17A by an ELISA (R & D Systems).
Histology and Immunohistochemistry
AAA specimens were immediately fixed in 10% formalin. After 24 hours, fixed samples were embedded in paraffin, and sections were stained by immunohistochemistry. Aortic sections were also stained with hematoxylin and eosin, and Verhoeff-Van Gieson for elastin. Immunohistochemical staining of mouse neutrophils and macrophages were performed using rat anti-mouse neutrophil (AbD Serotec, Raleigh, NC) and rat anti-mouse Mac-2 (Accurate Chem, Westbury, NY) primary antibodies, respectively. Alkaline phosphatase-conjugated anti-rat IgG (Sigma, St Louis MO) was employed as a secondary antibody. The signals were detected using Fast-Red (Sigma, St Louis MO). For CD3+ T cell (marker for both CD4+ and CD8+ T cells) immunostaining, the aorta sections (5µm) were dehydrated and incubated with 1% hydrogen peroxide followed by boiling in 1X unmasking solution (Vector Laboratories, Burlingame, CA) for 15 minutes and blocked with 10% serum. Immunostaining was performed with goat anti-mouse CD3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) using Vectastain ABC kit (Vector). After incubation with an avidin-biotin complex, immunoreactivity was visualized by incubating the sections with 3, 3-diaminobenzidine tetrahydrochloride (DAKO Corp, Carpinteria, CA) to produce a brown precipitate. Sections were then counterstained with hematoxylin. For smooth muscle α-actin (SM-αA) staining, the alkaline phosphatase-conjugated monoclonal anti-SM-αA antibody (Sigma, St Louis MO) was used. Images were acquired using 20X magnification by an Olympus microscope equipped with an Olympus digital camera, and ImagePro software.
Statistical Analysis
Experimental groups were compared using non-parametric statistical evaluation by 2-tailed Mann-Whitney U tests using GraphPad Prism 5 software. Pair-wise comparisons of groups are presented where P value less than 0.05 was considered statistically significant. Data are presented as mean±SEM.
Results
IL-23 and IL-17 Expression is Increased in Human AAA
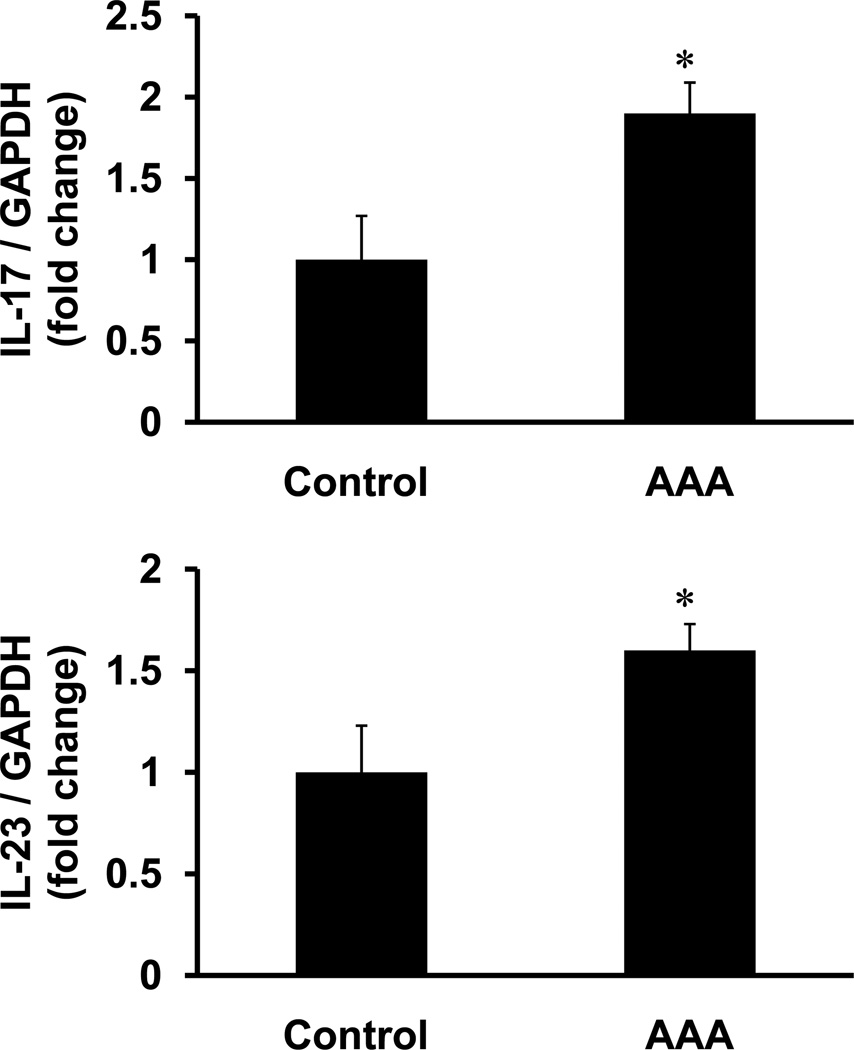
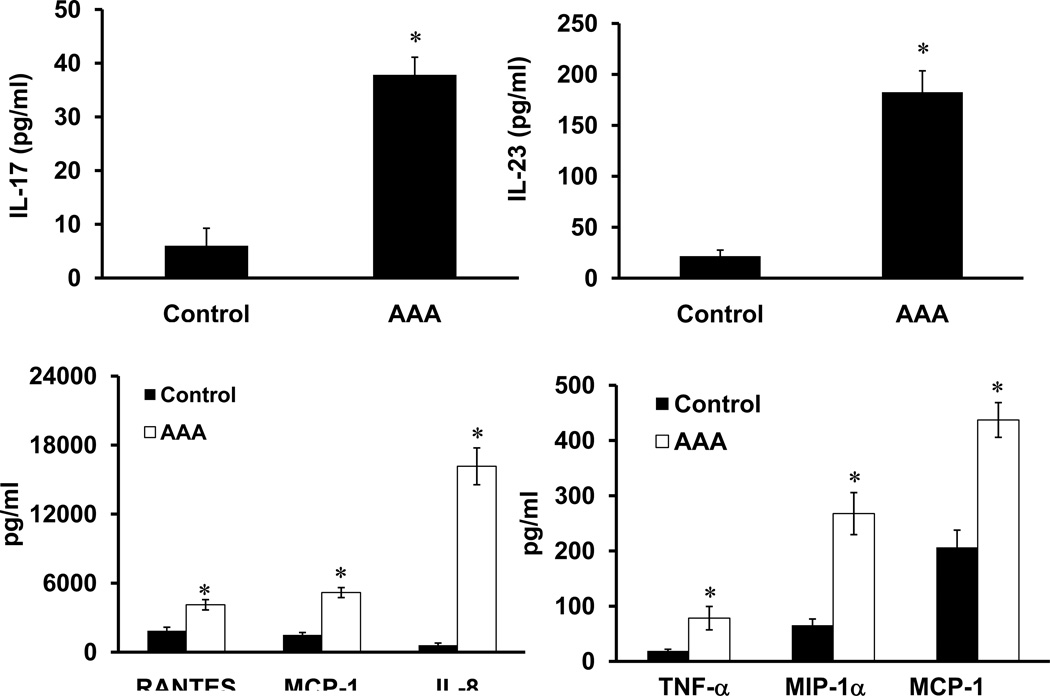
Gene expression of IL-17 and IL-23 was significantly increased in aortic tissue from AAA patients compared to controls (1.9±0.23 fold and 1.6±0.13 fold, respectively) (Figure 1). Protein levels of IL-17 (37.8±3.8 vs. 6.0±3.2 pg/ml) and IL-23 (182.5± 21.0 vs. 21.6± 6.0 pg/ml) were significantly elevated in aortic tissue from AAA patients compared to controls (Figure 2). A significant increase in the protein levels of RANTES, MCP-1, IL-8, TNF-α, MIP-1α, and IFN-γ was also observed in aortic tissue from AAA patients compared to controls (Figure 2).
Figure 1.
Gene expression of IL-17 and IL-23 are increased in human AAA. RT-PCR analysis of human aortic tissue demonstrated an increased expression of IL-17 and IL-23 in AAA patients (n=16) compared to controls (n=8). Results shown as fold change in gene expression normalized to GAPDH; *p< 0.05 vs. controls.
Figure 2.
Increased cytokine protein expression in human AAA. A significant, multifold increase in pro-inflammatory cytokine expression, including IL-17 and IL-23, was present in human aortic tissue from AAA patients (n=16) compared to controls (n=8). *p< 0.05 vs. controls.
IL-23 and IL-17 Modulates Inflammation and Contributes to AAA Formation
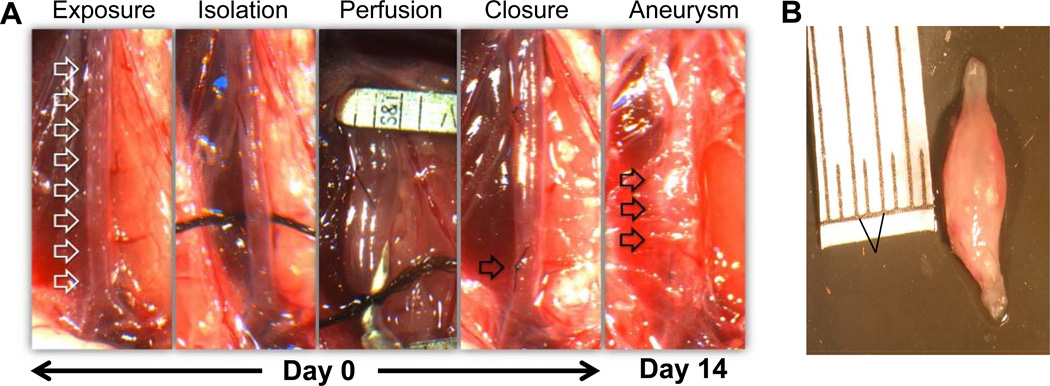
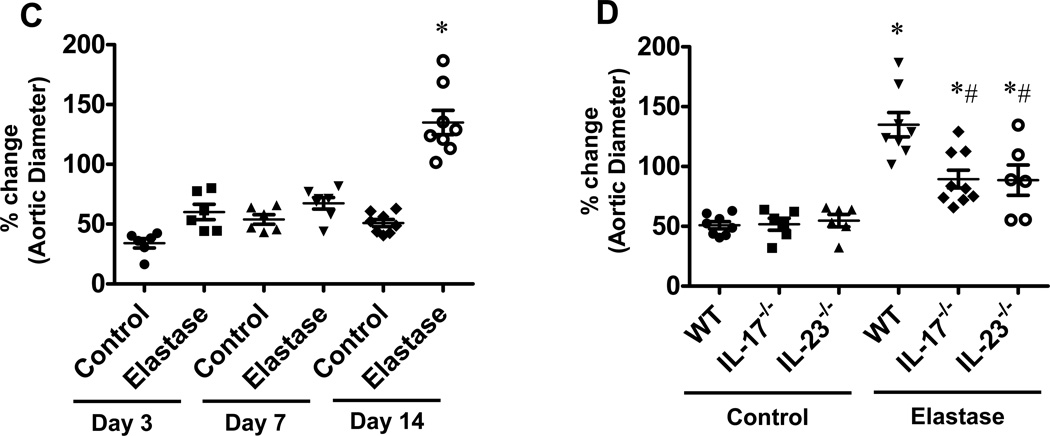
Using the elastase perfusion model, aortic diameter was measured in WT, IL-17−/− and IL-23−/− mice (Figure 3A and 3B). There was no significant difference in aortic diameter between elastase-perfused and heat-inactivated elastase-perfused (control) WT mice on days 3 and 7 (Figure 3C). However, AAA formation in WT mice after elastase perfusion was a consistent finding on day 14 with a mean aortic diameter increase of 141.1±16.1% vs. 51.1±4.1% (elastase-perfused vs. control WT mice, respectively) (Figure 3C). Importantly, AAA formation was significantly attenuated in elastase-perfused IL-17−/− (89.4±7.4%) and IL-23−/− (88.5±13.8%) mice compared to elastase-perfused WT mice (141.1±16.1%) on day 14, although not completely decreased to WT control levels (Figure 3D),. There was no significant difference in aortic diameter between control and elastase-perfused IL-17−/− and IL-23−/− mice compared to corresponding WT mice on days 3 and 7 (data not shown).
Figure 3.
AAA formation is attenuated in IL-17−/− and IL-23−/− mice. A. Infrarenal mouse aortas were perfused with elastase (0.4U/mL) or heat-incativated elastase (control), and aortic diameter was measured on days 3, 7 and 14. The abdominal aorta (Day 0, first panel, represented by white arrows) was significantly increased in diameter after elastase-perfusion of WT mice (Day 14, last panel, represented by black arrows). B. A representative AAA dissected from an elastase-perfused WT mouse on day 14. C. Elastase-perfused WT mice had no significant change in aortic diameters on days 3 and 7 compared to controls. On day 14, aortic diameter was significantly increased in elastase-perfused WT mice compared to controls. D. A significant decrease in aortic diameter occurred in elastase-perfused IL-17−/− and IL-23−/− mice compared to elastase-perfused WT mice. n=6–9 animals/group; *p<0.05 vs. corresponding controls; #p<0.05 vs. elastase-perfused WT mice.
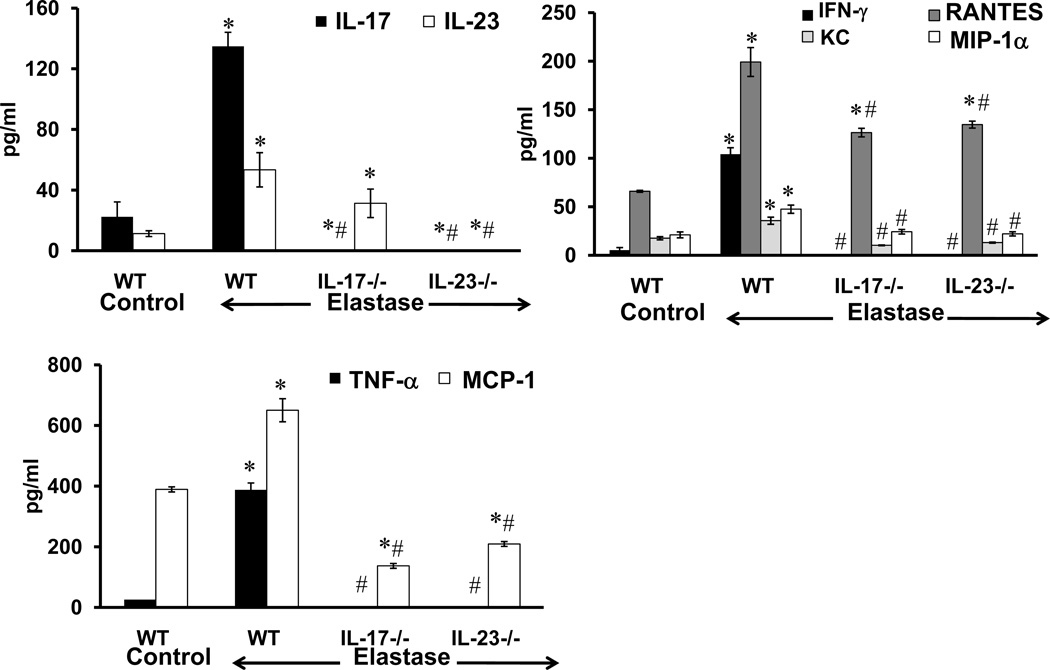
A significant increase in IL-17, IL-23, IFN-γ, RANTES, KC, MIP-1α, TNF-α and MCP-1 expression was observed in aortic tissue from elastase-perfused WT mice compared to controls on day 14 (Figure 4). More importantly, pro-inflammatory cytokines were significantly attenuated in aortic tissue from elastase-perfused IL-17−/− and IL-23−/− mice compared to elastase-perfused WT mice (Figure 4). IL-23 expression was not significantly decreased in IL-17−/− mice (Figure 4).
Figure 4.
Pro-inflammatory cytokine production is attenuated in elastase-perfused IL-17−/− and IL-23−/− mice. Cytokine expression was measured on day 14 in aortic tissue from WT, IL-17−/− and IL-23−/− mice which underwent control or elastase perfusion. A significant increase in IL-17 and IL-23 expression occurred in elastase-perfused WT mice compared to controls. A significant attenuation of IFN-γ, KC, MIP-1α, TNF-α and MCP-1 occurred in elastase-perfused aortic tissue from IL-17−/− or IL-23−/− mice compared to elastase-perfused WT controls. n=6–9 animals/group; *p<0.05 vs. WT control; #p<0.05 vs. elastase-perfused WT mice.
CD4+ T Lymphocytes Play a Key Role in AAA Formation via IL-17 Production
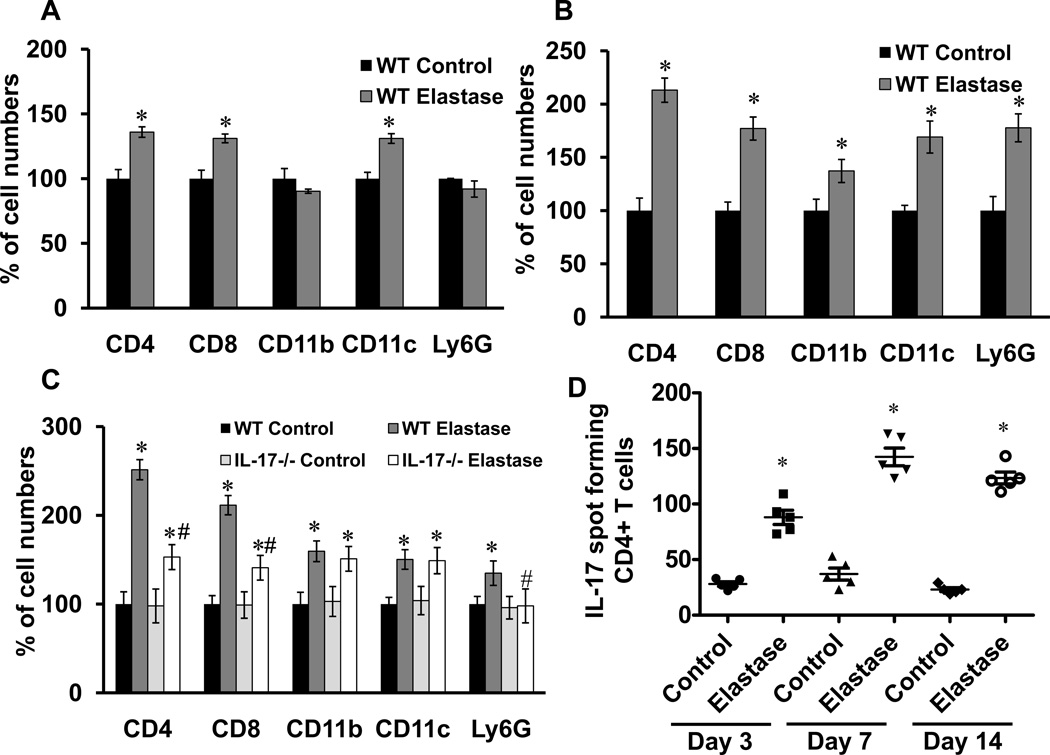
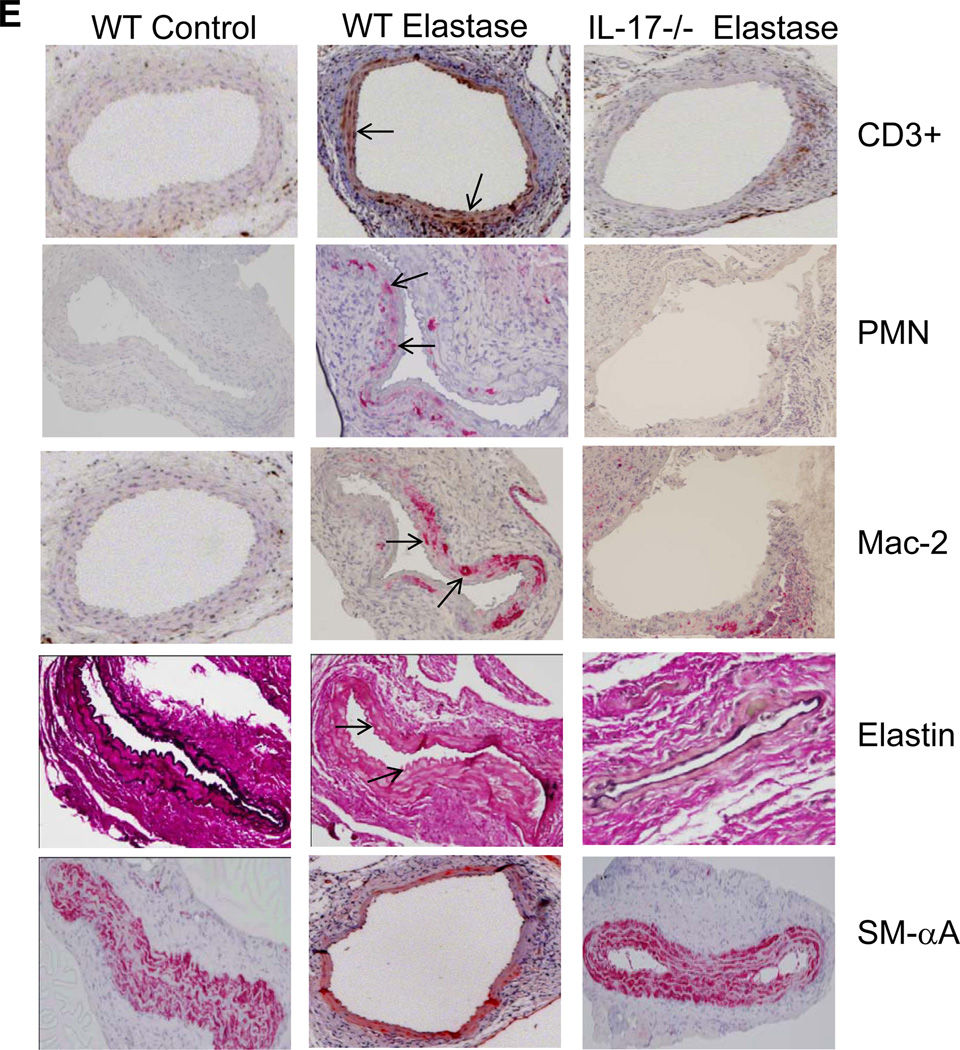
Cell infiltration in aortic tissue was compared between elastase-perfused and control WT mice on days 3, 7 and 14 by flow cytometry. A significant increase in CD4+ and CD8+ T cells as well as CD11c+ dendritic cells was observed in elastase-perfused WT mice compared to controls at day 3 (Figure 5A). Cellular infiltration peaked on day 7 and remained elevated on day 14 with a significant increase in T lymphocytes (CD4+ and CD8+), macrophages (CD11b+), dendritic cells (CD11c+) and neutrophils (Ly6G+) in elastase-perfused WT mice compared to controls (Figure 5B and 5C). On day 14, a significant attenuation of CD4+ and CD8+ T lymphocytes as well as neutrophils, but not macrophages or dendritic cells, occurred in aortic tissue from elastase-perfused IL-17−/− mice compared to elastase-perfused WT mice (Figure 5C). Furthermore, ELISPOT analysis demonstrated a significant increase in IL-17 production in CD4+ T cells from aortic tissue of WT mice after elastase perfusion compared to controls on days 3, 7 and 14 (Figure 5D). Furthermore, comparative histology and immunostaining of aortic tissue revealed a significant attenuation of inflammatory cell (CD3+ T cells, macrophages and neutrophils) infiltration, increase in smooth muscle α-actin expression and decrease in elastic fiber disruption in elastase-perfused IL-17−/− mice compared to elastase-perfused WT mice (Figure 5E).
Figure 5.
CD4+ T cell infiltration and IL-17 production is increased in aortas from elastase-perfused WT mice. Flow cytometry analysis of cell counts was performed on aortic tissue from control or elastase-perfused WT mice on days 3, 7 and 14. Results are shown as percentage of cell numbers compared to WT controls; n=4–5 mice/group; *p<0.05 vs. WT control, #p<0.05 vs. WT elastase. A. On day 3, a significant increase in CD4+ and CD8+ T cells as well as CD11c+ dendritic cells was observed in elastase-perfused WT mice compared to controls. B. On day 7, a significant increase in CD4+ and CD8+ T cells, CD11b+ (macrophages), CD11c+ (dendritic) and Ly6G+ (neutrophils) was observed in elastase-perfused WT mice compared to controls. C. On day 14, a significant increase in all cell populations remained significantly increased in elastase-perfused WT mice compared to controls. Elastase-perfused IL-17−/− mice had a significant attenuation in CD4+ and CD8+ T cells as well as Ly6G+ neutrophils compared to elastase-treated WT mice. D. ELISPOT analysis of IL-17-producing spot forming CD4+ T cells demonstrated a significant increase in CD4+ T cell-producing IL-17 cells in elastase-perfused aortic tissue from WT mice compared to controls on days 3, 7 and 14. E. Comparative histology and immunohistochemistry performed on day 14 indicates a significant decrease in CD3+ T cell, neutrophil (PMN) and macrophage (Mac-2) immunostaining, increase in smooth muscle cell α-actin (SM-αA) expression (arrows indicate areas of immunostaining), and decrease in elastic fiber disruption in aortic tissue (Verhoeff-Van Gieson staining for elastin) of elastase-perfused IL-17−/− mice compared to elastase-perfused WT mice. n=3–5/group; *p<0.05 vs. WT control.
IL-17 Modulates Aortic Smooth Muscle Cell Activation
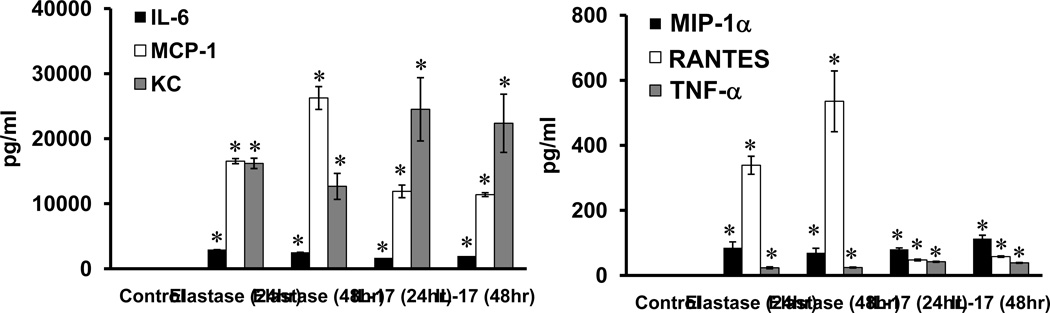
One feature of the pathogenesis of AAA formation is inflammation and vascular remodeling of aortic smooth muscle cells (ASMCs). To test if IL-17 can independently modulate the activation of ASMCs, primary ASMCs (1×106 cells) were transiently (5 min) exposed to elastase (0.4 U/mL). Cells were washed with PBS and fresh media was added. Cytokine measurement in culture supernatants was done after 24hr or 48 hr. ASMCs were also separately treated with recombinant IL-17 (10 ng/ml) for 24hr or 48hr and cytokine production in the supernatants was measured at endpoints. Elastase or IL-17 treatment of ASMCs significantly increased the production of IL-6, MCP-1, KC, MIP-1α and RANTES (Figure 6). There was no production of either IL-17 or IL-23 from ASMCs after elastase treatment (data not shown).
Figure 6.
IL-17 modulates ASMC activation. Transient treatment of ASMCs with elastase (5 min) or recombinant IL-17 (10ng/ml) was performed, and cytokine measurement was done after 24hr or 48hr. A significant increase in IL-6, MCP-1, KC, MIP-1α RANTES and TNF-α occurred in elastase-treated or IL-17-treated ASMCs compared to controls. n=8/group; *p<0.05 vs. controls.
Placental Mesenchymal Stem Cells Suppress Lymphocyte Activation, Attenuate IL-17 Production and AAA Formation
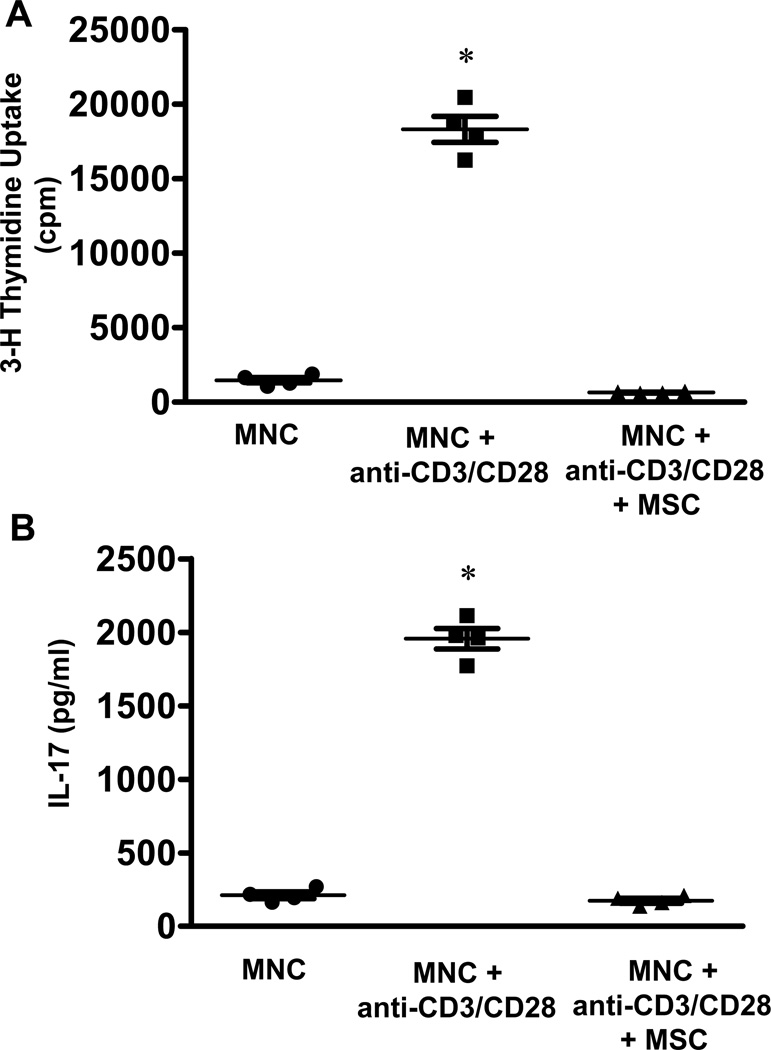
In vitro experiments demonstrated that activation of mononuclear cells (MNCs) by anti-human CD3 and CD28 resulted in significant cell proliferation compared to unstimulated MNCs (18,323.2±882.2 vs. 1475.7±182.0 cpm, respectively) (Figure 7A). MSCs co-cultured with MNCs suppressed the proliferation of activated MNCs (646.5±29.1 vs. 18,323.2±882.2 cpm, respectively). Furthermore, MSCs significantly attenuated IL-17 production by activated MNCs (163.5±27.8 vs. 1874.5±103.3 pg/ml, respectively) (Figure 7B).
Figure 7.
MSCs attenuate lymphocyte proliferation and IL-17 production in mixed lymphocyte reactions. A. MNCs activated by anti-CD3 and anti-CD28 show a significant increase in proliferation compared to unactivated MNCs, and was attenuated by MSC co-cultures, as measured by thymidine incorporation. B. Activated MNCs had a significant increase in IL-17 production compared to unactivated MNCs, which was attenuated by MSC co-cultures. n=4, *p<0.05 vs. all.
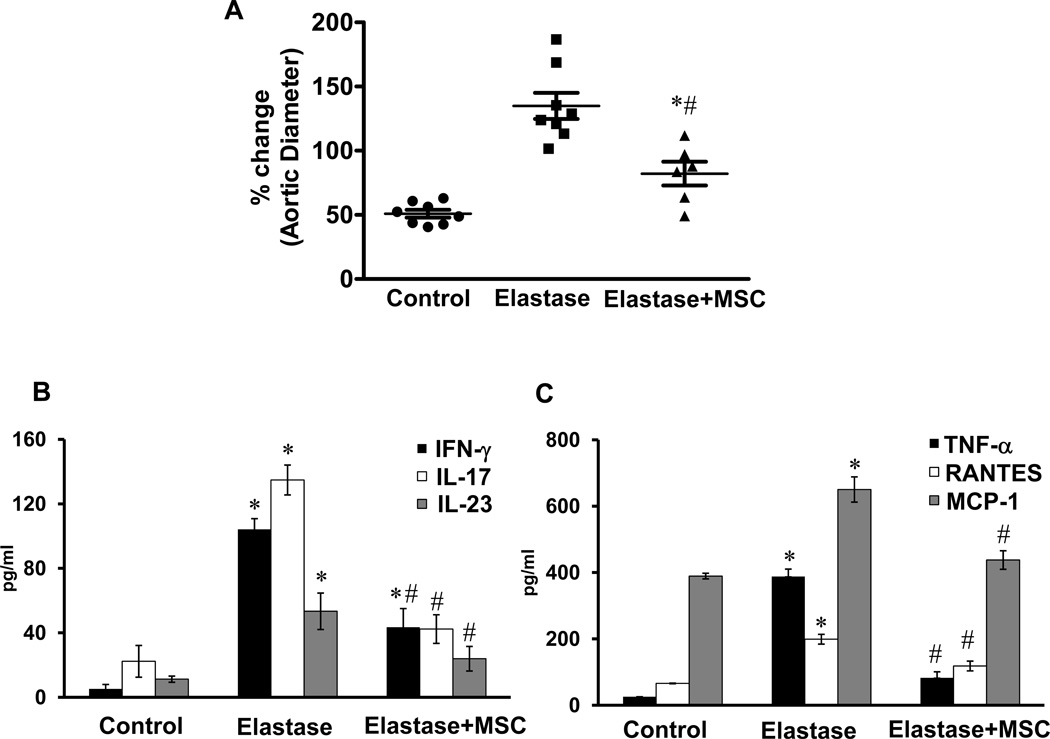
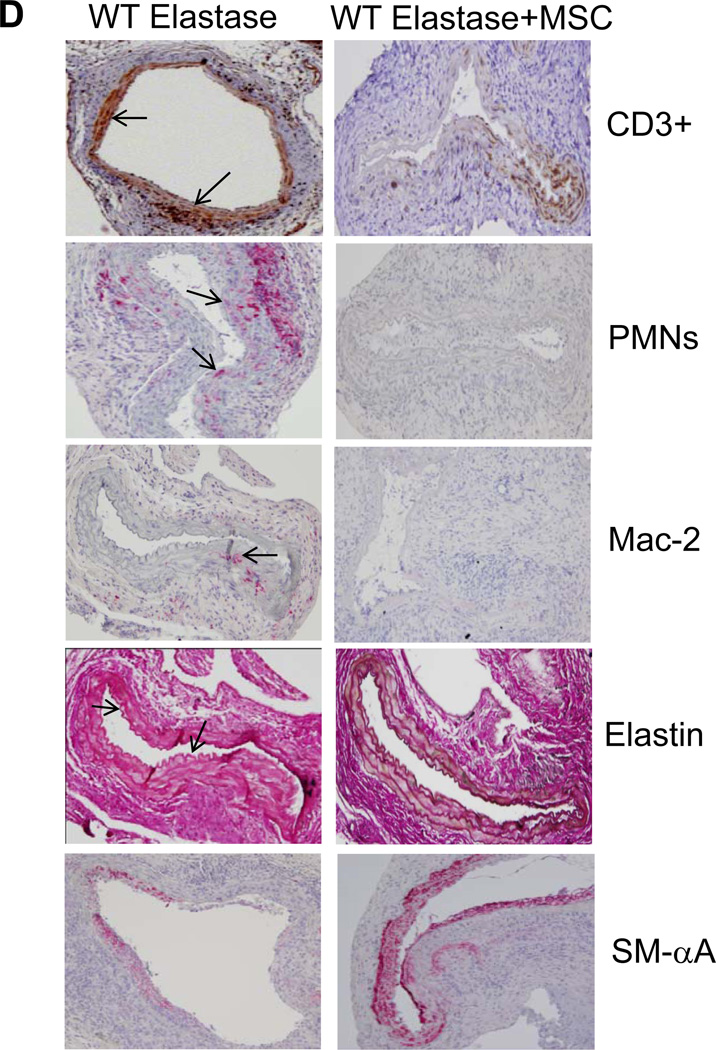
Based on these results, we tested the immunomodulatory effects of MSCs on AAA formation. Elastase-perfused WT mice were treated with MSCs (1×106 cells; intravenously) on day 1 and aortic diameter was measured on day 14. Aortic diameter was significantly attenuated in MSC-treated mice compared to untreated elastase-perfused WT mice (82.1±9.2% vs. 141.1±16.1%, respectively) (Figure 8A). Furthermore, aortic tissue from MSC-treated WT mice displayed a significant attenuation of pro-inflammatory cytokines, including IL-17, compared to elastase-perfused WT mice (Figure 8B and 8C). Also, comparative histology and immunohistological analysis of aortic tissue revealed a significant attenuation of inflammatory cell infiltration (CD3+ T cells, macrophages and neutrophils), increase in smooth muscle cell α-actin expression and decrease in elastic fiber disruption in MSC-treated elastase-perfused WT mice compared to elastase-perfused WT mice alone (Figure 8D). These results demonstrate that MSCs can attenuate AAA formation and IL-17 production.
Figure 8.
MSCs attenuate AAA formation. A. Aortic diameter measurement of elastase-perfused WT mice treated with MSCs showed a significant attenuation in AAA formation compared to elastase-perfused WT mice alone on day 14. B. Aortic tissue from elastase-perfused WT mice treated with MSCs showed a significant attenuation in IL-17 and IL-23 production compared to elastase-perfused WT mice alone. C. MSC treatment significantly attenuated pro-inflammatory cytokine/chemokine production in elastase-perfused WT mice. D. Comparative histology and immunohistochemistry performed on day 14 indicates that WT mice treated with MSCs have a significant decrease in CD3+ T cell, neutrophil (PMN) and macrophage (Mac-2) infiltration (arrows indicate areas of immunostaining), increase in smooth muscle cell α-actin (SM-αA) expression as well as decrease in elastic fiber disruption (Verhoeff-Van Gieson staining for elastin), compared to elastase-perfused WT mice. n=5–9 mice/group; *p<0.05 vs. control; #p<0.05 vs. elastase.
Discussion
This study establishes that IL-17 is a critical mediator of AAA formation in an elastase perfusion experimental model and that the source of IL-17 is CD4+ T cells. An increased expression of IL-23 and IL-17 was observed in aortic tissue from male AAA patients, and investigations into the importance of the IL-23/IL-17 axis were then conducted using in vivo and in vitro studies. Aortic diameter and pro-inflammatory cytokine production was significantly attenuated in aortic tissue of elastase-perfused IL-17−/− and IL-23−/− mice compared to WT mice. CD4+ T cells were noted to be one of the initial cell subsets to be significantly increased in aortic tissue during AAA and remained markedly elevated on days 3, 7 and 14, which also correlated with significant IL-17 production in WT mice after elastase perfusion compared to controls. In vitro studies confirmed that elastase or IL-17 treatment modulates activation of ASMCs. Furthermore, MSC treatment of WT mice after elastase perfusion significantly attenuated AAA formation as well as pro-inflammatory cytokine production, including IL-17. Immunomodulation of lymphocytes by MSC treatment was confirmed by in vitro studies which demonstrated a significant reduction in IL-17 production and lymphocyte proliferation. Taken together, these results demonstrate that CD4+ T cell produced-IL-17 plays a critical role in inflammation, ASMC activation and AAA formation, and that mitigation of IL-17 by MSC treatment offers significant protection from AAA formation.
Recent studies demonstrate an important role for T cell-produced IL-17 in models of chronic inflammation and leukocyte trafficking.6, 7, 21 IL-17 is largely produced by memory T cells and stimulate innate immunity and host defense.7 IL-17A production by T lymphocytes can be regulated by IL-23 independent of T cell receptor activation and has been shown to be a potent mediator of neutrophil and macrophage recruitment in various chronic inflammatory models.21 Macrophages or dendritic cells are known to be prominent sources of IL-23, which has been shown to regulate CD4+ T cell-produced IL-17 in various models.22
IL-17 has pro-inflammatory properties and acts on a broad range of cell types to induce the expression of various cytokines, chemokines and metalloproteinases.7 Recent studies have shown a definitive role of IL-17 in the promotion of vascular inflammation and atherosclerosis.21, 23 The current study identifies a key role of the IL-23/IL-17 axis in AAA formation by showing decreased aneurysm phenotype as well as overall reduction in pro-inflammatory cytokine/chemokine production in IL-23−/− and IL-17−/− mice. Although both IL-23−/− and IL-17−/− mice were significantly protected from AAA formation, however the expression of IL-17 was significantly attenuated in IL-23−/− mice, but not vice versa, thereby confirming the proinflammatory effector role of IL-17 in our model. Of particular interest was the significant attenuation of TNF-α, IFN-γ and MCP-1 in elastase-perfused IL-17−/− mouse aortas. These cytokines have been shown to mediate aneurysm formation in animal studies and are upregulated in human AAA.3, 4, 24 The regulation of these inflammatory cytokines by IL-17 suggests that CD4+ T cell-produced IL-17 is an early and upstream mediator of the inflammatory cascade involved in AAA.
The regulation of inflammatory cytokines and MMP suppression by MSC treatment of aortic aneurysm has been previously reported.10 The suppression of T cells, dendritic cells and macrophages by MSCs has also been demonstrated previously.25 Based on our findings that AAA formation in our mouse model was IL-17 dependent, and that MSCs suppressed both MNC proliferation and IL-17 production, we assessed the potential of MSC therapy in vivo. Here, MSC treatment significantly attenuated AAA formation and IL-17. One potential mechanism for MSC-mediated suppression of the excess immunopathologic signaling in the aneurysmal vascular wall could be via a paracrine manner involving soluble factors like TGF-β, hepatocyte growth factor or prostaglandin E2. Alternatively, the proliferation and differentiation of MSCs into smooth muscle cells and attenuation of vascular inflammation may contribute to phenotypic restoration of abdominal aorta after aneurysm formation. Further studies characterizing the regenerative capabilities of MSCs in the injured aortic tissue are currently under investigation.
This study is the first to document the importance of IL-17 in experimental AAA formation. Although previous studies have shown the importance of various cytokines in aneurysm development, the significance of T cell produced-IL-17 in the modulation of other cytokines and regulation of inflammation as well as aortic smooth muscle activation is a key finding regarding the molecular mechanisms associated with AAA pathogenesis. The multiple facets of IL-17-mediated regulation of inflammation, aortic smooth muscle cell apoptosis and vascular remodeling makes IL-17 a potential target for treatment of aortic aneurysms. One such treatment strategy could potentially be via mesenchymal stem cells and further studies are needed to establish their therapeutic potential.
Acknowledgements
The authors would like to thank Rupande Tripathi for isolation of primary aortic smooth muscle cells. We also thank Sebastien Coquery and the University of Virginia Flow Core facility for help with the flow cytometry experiments and Dr. George Stukenborg for help with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts to disclose.
References
- 1.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 3.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 4.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 6.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 7.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Galle C, Schandene L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol. 2005;142:519–527. doi: 10.1111/j.1365-2249.2005.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 10.Hashizume R, Yamawaki-Ogata A, Ueda Y, Wagner WR, Narita Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J Vasc Surg. 2011;54:1743–1752. doi: 10.1016/j.jvs.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull IC, Hadri L, Rapti K, Sadek M, Liang L, Shin HJ, Costa KD, Marin ML, Hajjar RJ, Faries PL. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J Surg Res. 2011;170:e179–e188. doi: 10.1016/j.jss.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 17.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, Eagleton MJ, Henke PK, Wakefield TW, Myers DD, Stanley JC, Upchurch GR., Jr L-selectin- mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112:241–247. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]
- 18.Colonnello JS, Hance KA, Shames ML, Wyble CW, Ziporin SJ, Leidenfrost JE, Ennis TL, Upchurch GR, Jr, Thompson RW. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J Vasc Surg. 2003;38:138–146. doi: 10.1016/s0741-5214(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 19.Sharma AK, Lapar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural Killer T Cell-derived IL-17 Mediates Lung Ischemia-Reperfusion Injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park TS, Gavina M, Chen CW, Sun B, Teng PN, Huard J, Deasy BM, Zimmerlin L, Peault B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20:451–463. doi: 10.1089/scd.2010.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moehle CW, Bhamidipati CM, Alexander MR, Mehta GS, Irvine JN, Salmon M, Upchurch GR, Jr, Kron IL, Owens GK, Ailawadi G. Bone marrow-derived MCP1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J Thorac Cardiovasc Surg. 2011;142:1567–1574. doi: 10.1016/j.jtcvs.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]