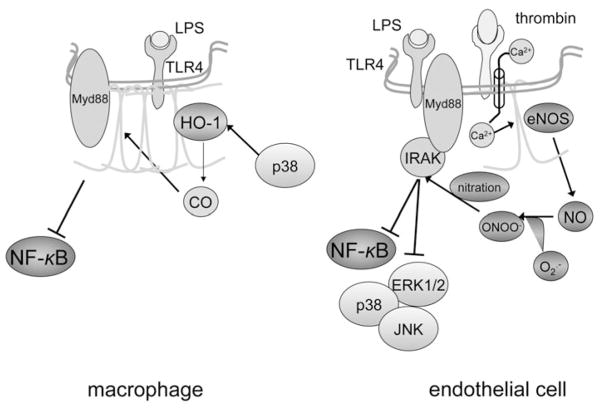
Figure 4.
Modulation of Toll-like-receptor-dependent signaling pathways by caveolin-1. Data obtained primarily on macrophages (left panel) favor a suppressive function of caveolin-1 on Toll-like receptor-4 (TLR-4) signaling. This is accomplished on several levels, starting by physical association with the receptor within caveolae, which presumably diminishes binding to adaptor protein MyD88, a required step for propagation of the TLR-4 signal upon docking of lipopolysaccharide (LPS). Activation of heme oxygenase-derived CO production by p38, which is expected to occur following TLR-4 ligation by LPS, is an additional mechanism to turn off the TLR-4 signal (adapted from Wang et al 2009). A seemingly opposing function of caveolin-1 has been advocated in knockdown experiments in endothelial cells (right panel). Excessive free radical (nitric oxide-NO and superoxide-O2−) production due to lack of caveolin-1-mediated endothelial NO synthase (eNOS) inhibition may lead to nitration of interleukin-1-associated receptor kinase (IRAK)-4, an important intermediate between TLR-4 and its effector molecules nuclear factor-κ (NF-κB) and mitogen-activated kinases (p38, ERK1/2, JNK). Hypothetically, this mechanism could take effect in situations in which eNOS is uncoupled from caveolin-1, as may occur in the presence of vasoactive mediators including thrombin. In this case, dual activation of eNOS due to Ca++ influx and dissociation from caveolin-1 could result in production of large amounts of free radicals and tyrosine nitration via the reactive metabolite peroxynitrite (ONOO−). A color version of this image is available online at www.landesbioscience.com/curie.