Figure 4. X-ray structure of a benzothiazole-FLuc co-crystal.
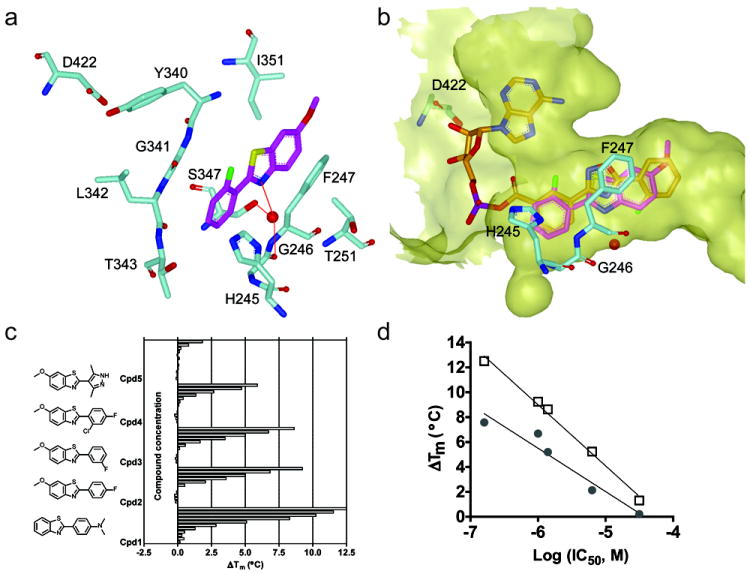
a) Interactions of a benzothiazole (magenta) within the D-LH2 pocket of FLuc. Aromatic stacking interactions between the benzothiazole core and F247 are observed. A water-mediated H-bond was formed between benzothiazole nitrogen and G246 backbone carbonyl oxygen and S347 hydroxyl, as shown by the red dotted line. Protein residues are shown in cyan; supported by Table S1 and Figure S4. b) Overlay with of the benzothiazole (magenta) with the FLuc-bound structure of PTC124-AMP (PDB: 3IES; orange) showing occupation of the D-LH2 pocket by the benzothiazole with no overlap into the AMP binding region of FLuc. The binding pocket was depicted as semi-transparent yellow surface. These figures were prepared with the program VIDA (OpenEye Scientific Software). c) Representative simple benzothiazoles and example thermal shift data assayed in the presence of 2 mM ATP. d) The ΔTm obtained at 100μM compound in the presence (open squares) or absence (solid circles) of 2 mM ATP plotted against the potency of each benzothiazole (shown in c).
