Abstract
We investigated the ability of transgenic torenia (Scrophulariaceae) plants to resist fungi and arthropod herbivores. Torenia hybrida cv. Summerwave Blue was manipulated to produce Arabidopsis agmatine coumaroyltransferase (AtACT). This catalyses the last step in the biosynthesis of hydroxycinnamic acid amides (HCAAs) which function in defence. Transgenic plants accumulated substantial HCAAs, predominantly p-coumaroylagmatine, and the HCAAs were isomerized from the trans-form to the cis-form in planta. The transgenic line, accumulated the highest amount of endogenous HCAAs (CouAgm at 32.2 µM and total HCAAs at 47.5 µM) and this line was resistant to the necrotrophic fungus, Botrytis cinerea. There was no resistance, however, in their wild-type progenitors or in other transgenic lines accumulating low HCAA amounts. In contrast, the transformants were not significantly resistant to three representative herbivores, Frankliniella occidentalis, Aphis gossypii, and Tetranychus ludeni.
Hydroxycinnamic acid amides (HCAAs) are antifungal substances, some of which have been shown to serve as phytoalexins synthesized de novo by plants in response to infection by incompatible pathogens1. HCAAs have been observed in several plant species, including members of the Poaceae1, Solanaceae2 and Brassicaceae3. They play roles in acquired immunity by suppressing the elongation of hyphae4,5,6 and limiting the entry of pathogens by being deposited in cell walls causing physical blockage or increasing leaf toughness2,7,8. HCAA-derived polymers have been reported as components of suberin (a highly hydrophobic substance that prevents water from penetrating the tissue) that has accumulated in mechanically wounded potato tubers9.
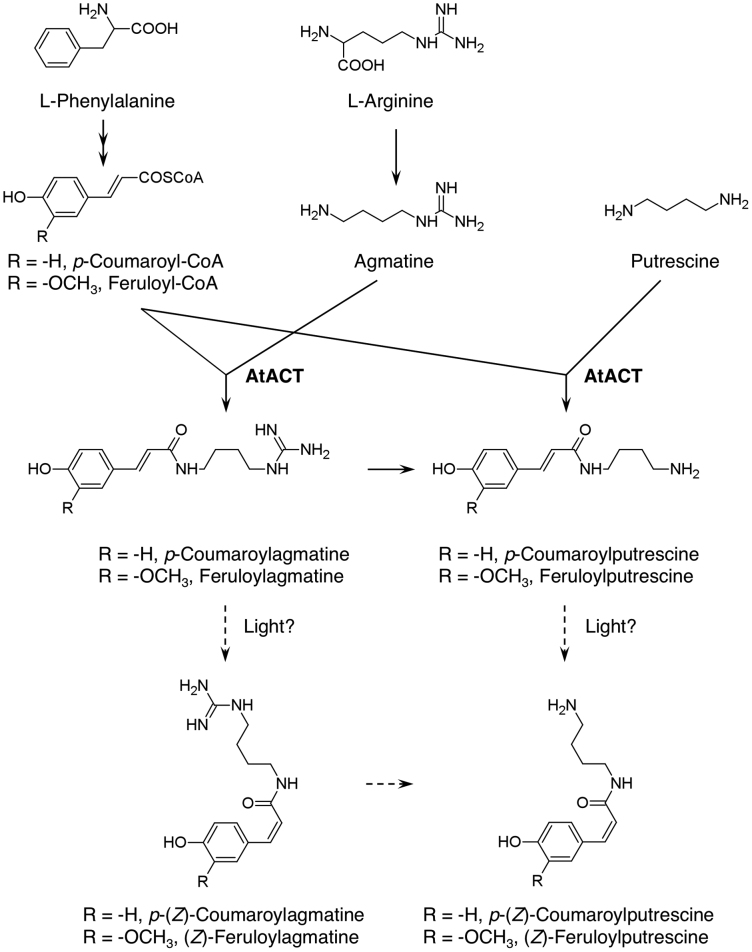
HCAAs are formed by the condensation of various biogenic amines with hydroxycinnamic acids derived from phenylalanine via the phenylpropanoid pathway. The genes involved in HCAA biosynthesis was first isolated from elicited cell cultures of carnation as anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT), which catalyses the condensation reaction between hydroxycinnamoyl-/benzoyl-CoA and anthranilate to form dianthramides10. In vitro, benzoyltransferase shows narrow substrate specificity for anthranilate but accepts a variety of aromatic acyl-CoAs10. Agmatine coumaroyltransferase (ACT), which catalyses the biosynthesis of antifungal hydroxycinnamoylagmatine derivatives, was isolated from etiolated barley seedlings11. Both native and recombinant ACT was highly specific for agmatine as an acyl acceptor and had the highest specificity for p-coumaroyl-CoA among various acyl donors11. ACT is classified in the BAHD acyltransferase family12 and is predicted to locate in the cytosol11. It was recently isolated from Arabidopsis, in which it mainly formed p-coumaroylagmatine (CouAgm) (81.2% of total product) and minor amounts of other HCAA products (feruloylagmatine [FerAgm, 3.7%], p-coumaroylputrescine [CouPtr, 14.6%] and feruloylputrescine [FerPtr, 0.5%])3 (Fig. 1). An AtACT T-DNA insertion line was deficient in accumulation of all the above HCAA products and was much more susceptible to Alternaria brassicicola infection than wild-type (WT) plants3.
Figure 1. Pathways of HCAA biosynthesis.
The catalytic function of Arabidopsis agmatine coumaroyltransferase (AtACT) is shown.
Torenia is an annual or perennia plant in the Scrophulariaceae, and is grown as an ornamental summer bedding plant. Hybrids have been produced in the last few decades, resulting in various floral colours ranging from white with yellow throats to blue, cobalt, lavender and violet. Torenia is also an experimental plant with several useful characteristics, such as ease of genetic transformation, differentiability of adventitious structures, applicability for in vitro fertilization, and small genome size, comparable with that of Arabidopsis13. Since a simple and efficient transformation system has been established for torenia, various transformation studies targeting modification of ornamental characteristics have been conducted13. To assess the ability of HCAA compounds to control pests and diseases of torenia, we produced transgenic plants which constitutively express the AtACT gene and tested their defences against pathogens and arthropod herbivores.
Results
Generation of transgenic torenia plants producing HCAAs constitutively
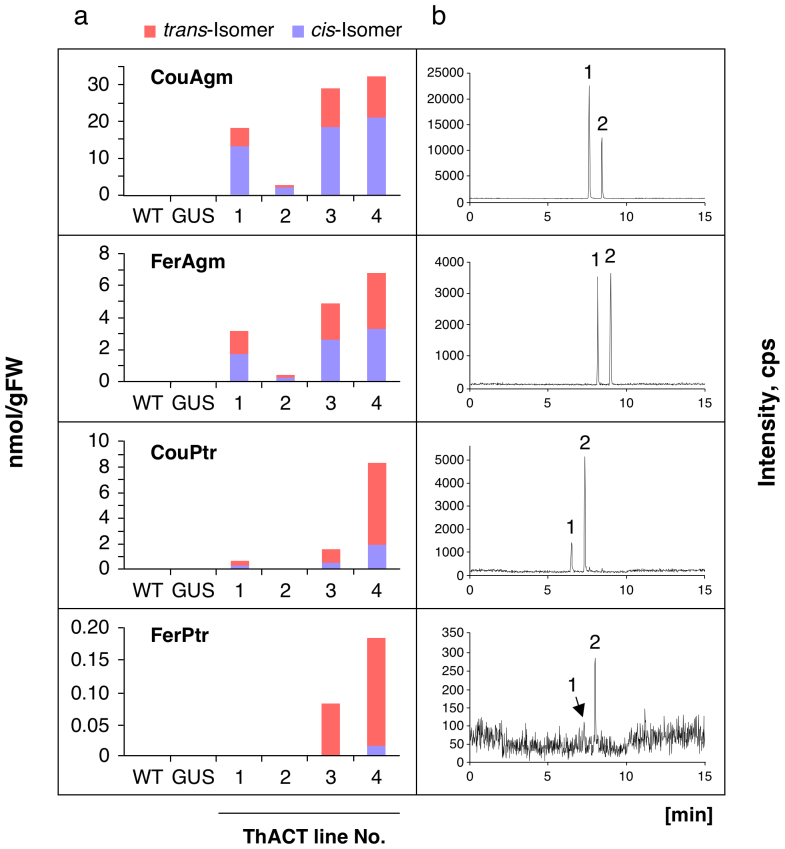
Results of four representative lines (called ThACT) are presented (Fig. 2). CouAgm accumulated in leaves at between 2.6 (ThACT2) and 32.2 nmol g–1 FW (ThACT4), whereas WT and GUS-transgenic control plants showed no detectable accumulation. The other HCAA compounds were accumulated in only trace amounts, peaking in ThACT4 at 8.3 nmol CouPtr g–1 FW (17.6% of the total HCAA product), 6.8 nmol FerAgm g–1 FW (14.3%) and 0.2 nmol FerPtr g–1 FW (0.4%). The cis-isomers of all the HCAAs in the transgenic torenia leaves were present in much higher amounts than those from the native AtACT protein and from recombinant protein produced in Escherichia coli and fungus-infected Arabidopsis leaves3. The trans-isomers of phenylpropanoid products are likely cis-isomerized by light14.
Figure 2. Accumulation of HCAAs in the leaves of wild-type (WT), GUS-transgenic control and ThACT plants.
(a) Means of trans- and cis-isomers of HCAA products in leaves (n = 4). (b) Representative HPLC-MS/MS profiles of HCAAs. Peaks 1 and 2 correspond to trans- and cis-isomers. The monitored mass transitions (Q1/Q3) were m/z 277.2/147.2 for CouAgm, 307.2/177.2 for FerAgm, 235.2/147.2 for CouPtr, and 265.2/177.2 for FerPtr.
All the ThACT lines exhibited trans-gene (AtACT) expression in the leave, whereas WT and GUS-transgenic control plants did not (SI, Supp. Fig. 1 ).
Immunity to fungi
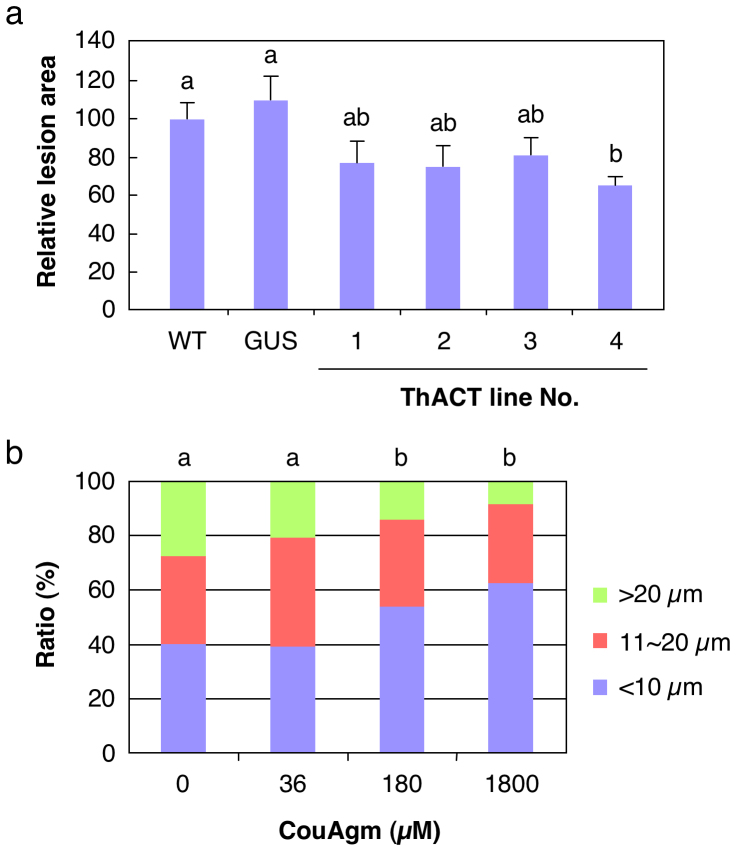
ThACT4 showed resistance to Botrytis cinerea, with clearly smaller lesions than on WT and the GUS-transgenic control leaves 48 h after infection (F = 3.710, df = 5, P < 0.01, ANOVA followed by Tukey-Kramer HSD test, P < 0.05; Fig. 3a). However, the three other lines were not significantly more resistant than WT and the GUS-transgenic control leaves, although they had smaller lesions than did the WT and the GUS-transgenic control leaves (P > 0.05). Higher concentrations of CouAgm (180 and 1800 µM) significantly inhibited the germination and development of conidial germ tubes (0 vs 180 µM or 1800 µM; χ2-test weighted by the Bonferroni correction (df = 1, P < 0.05/n = 0.008, n = 6: the number for all combinations of the 4 groups; Fig. 3b). But 36 µM, which approximated the endogenous level in ThACT4, was ineffective (0 vs 36 µM; χ2-test, df = 1, P = 0.106; Fig. 3b). Nevertheless, there seemed to be fewer well grown germ tubes (>21 µm) than in the control.
Figure 3. Inhibitory effects of HCAAs on B. cinerea development.
(a) B. cinerea lesions on the leaves of wild-type (WT), GUS-transgenic control and ThACT plants 48 h after inoculation. Means (± > + SE) of relative lesion areas with different letters are significantly different (n = 47−77; ANOVA followed by Tukey-Kramer HSD test, P < 0.05). (b) Dose effect of exogenous CouAgm on germination of B. cinerea conidia. The conidia were grouped by germ tube length (0–10, 11–20, and >21 µm) to evaluate how well they grew. Means with different lower-case letters are significantly different (n = 222−302, χ2-test weighted by the Bonferroni correction, df = 1, P < 0.05/n = 0.008, n = 6: the number for all combinations of the 4 groups).
Resistance to arthropod herbivores
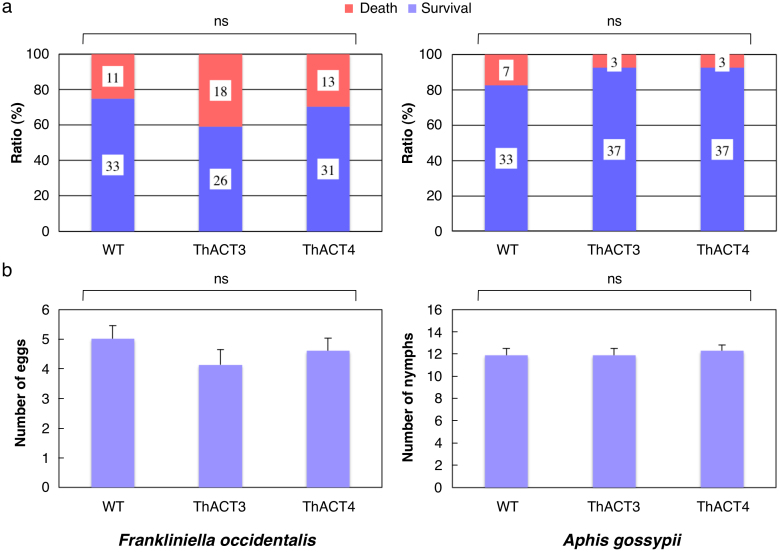
All three herbivores survived and reproduced equally well on WT and on the lines, ThACT3 and ThACT4 (Frankliniella occidentalis survival: GLM-test, χ2 = 2.697, df = 2, P = 0.26, Fig. 4a; oviposition: ANOVA, F = 0.663, df = 2, P = 0.518, Fig. 4b; Aphis gossypii survival: χ2 = 2.604, df = 2, P = 0.272; nymphs: F = 0.119, df = 2, P = 0.888; Tetranychus ludeni survival: GLM-test, χ2 = 3.245, df = 2, P = 0.197, see SI, Supp. Fig. 2a; oviposition: F = 0.663, df = 2, P = 0.518, see SI, Supp. Fig. 2b).
Figure 4. Effect of HCAAs on arthropod herbivores.
(a) Survival rate of adult female thrips (F. occidentalis) and aphids (A. gossypii) on the leaves of wild-type (WT), ThACT3 and ThACT4 lines 3 days after inoculation. ns, P > 0.05, GLM-test. (b) Total number of eggs or nymphs (means ± > + SE) produced during the inoculation period. ns, df = 2, P > 0.05, ANOVA.
Discussion
Botrytis is a common fungal disease of torenia leaves15. The constitutive accumulation of HCAAs in ThACT4 leaves (CouAgm at 32.2 µM; total HCAAs at 47.5 µM) suppressed B. cinerea lesion development, and the addition of extra CouAgm showed a dose effect at 180 and 1800 µM (Fig. 3b). Similar levels were detrimental to Monilinia fructicola, in which 200 µM CouAgm decreased the germination of spores by 24%, and 410 µM by 78%16. Moreover, CouAgm at >1000 µM inhibited the germination of spores and the elongation of hyphae of Alternaria brassicicola3. It is noteworthy that the endogenously produced CouAgm in ThACT4 was much less but nevertheless conferred tolerance to B. cinerea (Figs. 2a and 3a). Arabidopsis leaves inoculated with A. brassicicola accumulated CouAgm at 85 nmol g–1 FW after 3 days, but the same level applied exogenously was not sufficiently anti-fungal3. The difference might be explained by potential synergism with other HCAAs that accumulate, as seen with resveratrol, a phytoalexin with antioxidant activities, and curcumin, another phytochemical antioxidant, which showed great synergistic potential in antioxidant activity17. Thus, low levels of CouAgm in leaves may control fungi more effectively in the presence of synergists. In addition, since HCAAs, including CouAgm, have been found to be localized in cell wall fractions in various plant species3,18,19, a rich store of HCAAs in cell walls might be detrimental to fungal development.
Although ThACT3 leaves predominantly accumulated CouAgm at 29.9 µM, an amount comparable to that in ThACT4 (Fig. 2a), they were not clearly resistant to the fungi (Fig. 3a). This was probably due to an insufficient, below threshold, accumulation of endogenous total HCAAs, albeit a sufficient accumulation of CouAgm, in ThACT3 leaves. CouPtr was clearly accumulated much less in ThACT3 than in ThACT4 (Fig. 2a). It should be assessed, therefore, whether these HCAA products, except CouAgm, sustain anti-fungal activity.
In contrast to their anti-fungal activity, the HCAAs in the transgenic plants had no significant effects on the arthropod herbivores. Little is known about the effects of HCAAs on arthropod herbivores. However, HCAAs of tyramine and dopamine20 and phenolic acid derivatives and HCAAs of serotonin dimers21,22 have shown notable antioxidant activity. Since these HCAAs decreased membrane lipid peroxidation levels and induced plant defence responses23,24, we expected that the HCAAs in the ThACT lines would affect the herbivores. Instead, the transgenic plants had no detrimental effects on F. occidentalis, A. gossypii or T. ludeni (Fig. 4 and see SI, Supp. Fig. 2 ). Nevertheless, the HCAAs might affect bacterial and fungal pathogens transmitted via F. occidentalis and A. gossypii25,26, indirectly affecting these herbivores.
Remarkable advances in torenia research in recent years have made this garden plant notable as a model plant for genetic engineering aimed at studying ornamentation27,28 and pest control29. Future trials using transgenic torenia plants will aim at providing new insights into plant engineering, pest control and ecology.
Methods
Generation of transgenic torenia plants
We generated transgenic plants that constitutively biosynthesize HCAAs. We prepared gene constructs encoding Arabidopsis agmatine coumaroyltransferase (AtACT), which catalyses the last step in the biosynthesis of the HCAAs (Fig. 1), downstream of the constitutive 35S cauliflower mosaic virus (CaMV) promoter, and generated a set of independent transgenic torenia lines.
The full-length coding region of AtACT (At5g61160) was inserted into the GFP reporter gene site of the binary vector pSMABR35SsGFP, in which the selectable marker gene bar was replaced by the kanamycin resistance gene nptII30. The resulting plasmid, pSKAN-AtACT, was transformed into Agrobacterium tumefaciens strain EHA101 by electroporation. Torenia was transformed as described31. The A. tumefaciens strain carrying the binary plasmid pBI121 (containing the uidA gene coding for β-glucuronidase [GUS]), was transformed as a control. In brief, leaf fragments of in vitro–grown torenia plants (Torenia hybrida = T. fournieri × T. concolor cv. Summerwave Blue) were cocultured with Agrobacterium for 7 days at 22°C in the dark. Kanamycin-resistant shoots were regenerated by culturing on medium containing 300 mg L–1 kanamycin at 25°C under a 16-h photoperiod. Since torenia is a heterozygous plant developed by a combination of extensive hybridization and mutation breeding, it is difficult to obtain homozygous lines13. So the resulting transgenic plants (T0) were maintained in vitro by subculturing every month until use, under sterile conditions in a plant box containing 1/2 Murashige and Skoog medium32 supplemented with 3% sucrose and 0.2% gellan gum in a growth chamber at 25°C (160 µE m–2 s–1 with a 16-h photoperiod).
Plants and pests
Plants were maintained under sterile conditions as above. To prepare potted plants, we rooted herbaceous cuttings in soil, one to a plastic pot, in a climate-controlled room (25°C, 80 µE m–2 s–1, 16-h photoperiod) for 3 to 5 weeks until use.
For inoculation with B. cinerea (strain IuRy-1), a suspension of conidia (5 µL, 105 mL–1 in 2.5% glucose) was placed on the center of upper surface of a leaf, and the plants were covered with a plastic sheet to hold the humidity. The plants were placed in the dark at 25°C for 48 h. Leaf lesions <1.0 mm2 were not included in the analysis as these were caused by unsuccessful colonization.
Western flower thrips (F. occidentalis) were maintained on both pollen (of C. sinensis > Camellia sinensis) and seeds (of Vicia faba) in rearing cages in a climate-controlled room (22°C, 60 ± 10% relative humidity [RH], 16-h photoperiod)33. Female adults 3–7 days after emergence were used for experiments. Cotton aphids (Aphis gossypii >A. gossypii) were reared on eggplants (Solanum melongena cv. Senryo-nigou) in a climate-controlled room (15 > 22°C, 60 ± 10% RH, 13 > 16-h photoperiod). Female adults 3–5 days after emergence were used for experiments. Spider mites (T. ludeni) were reared on kidney bean plants (Phaseolus vulgaris cv. Nagauzuramame) in a climate-controlled room (22°C, 60 ± 10% RH, 16-h photoperiod). Adult female mites 3–7 days after the final moulting were used for experiments.
Chemical analysis
A piece of torenia leaf (25–50 mg fresh weight) was extracted with 10 volumes of absolute methanol at 25°C for 24 h. HCAAs were analysed by high-performance liquid chromatography (HPLC) – tandem mass spectrometry on an API 3000 chromatograph (Applied Biosystems, Foster City, CA, USA) in multiple reaction monitoring mode with a solvent gradient of 0.01% trifluoroacetic acid (TFA)–acetonitrile (95:5, v/v) at 0 min to 0.01% TFA–acetonitrile (70:30, v/v) at 10 min (column, Mightysil RP-18 GP 3 µm [Kanto Chemical, Tokyo, Japan], 2 mm × 100 mm; flow rate, 0.2 mL min−1; temperature, 40°C). The monitored mass transitions (Q1/Q3) were m/z 277.2/147.2 for CouAgm, 307.2/177.2 for FerAgm, 235.2/147.2 for CouPtr, and 265.2/177.2 for FerPtr.
Quantitative reverse transcription (RT)-PCR
Total RNA was isolated from leaf tissues using a Qiagen RNeasy Plant Mini Kit and an RNase-Free DNase Set (Qiagen > Qiagen, Hilden, Germany) following the manufacturer's protocol. First-strand cDNA was synthesized using a PrimeScript RT reagent Kit (Takara, Japan > Takara, Otsu, Japan), and 0.5 µg of total RNA at 37°C for 15 min. Real-time PCR was carried out on an Applied Biosystems 7300 Real-Time PCR System using Power SYBR Green Master Mix (Applied Biosystems), cDNA (1 μl from 10 μl of each RT product pool), and 300 nM primers. The following protocol was followed: initial polymerase activation 2 min at 50°C, and 10 min at 95°C, 40 cycles of 15 s at 95°C, and 60 s at 60°C. PCR conditions were chosen by comparing threshold values in a dilution series of the RT product, followed by non-RT template control and non-template control for each primer pair. Relative RNA levels were calibrated and normalized with the level of ACT3 (AB330989) mRNA. The primers used were as follows: AtACT (5′-TCGGGACTTGTGTCTTAGCC-3′ and 5′-CCCGTTTACGACCAACAACT-3′) and ACT3 (5′-CAACTGCAGAGCGTGAAATC-3′ and 5′-ATCATCGATGGCTGGAAAG-3′).
Effects of CouAgm on germination of B. cinerea conidia
B. cinerea grown on potato sucrose agar was exposed to black light (Panasonic FL10BL-B [10W]) for 1 week to promote the formation of conidia. Conidia were collected in sterilized water, filtered through one layer of facial tissue, centrifuged at 500 ×g for 3 min, and washed twice with sterilized water. The conidia were suspended at 2.6 × 106 mL–1 in malt extract broth containing 0.1% agar and a known concentration of CouAgm. The suspension (0.1 mL) was spread on an agar-coated plastic plate (1.1 cm2, 1.1 mm thickness), and incubated at 25°C for 4 h in dark at 100% RH. Germination was observed on three independent plates.
Effects on resistance to arthropod herbivores
Leaf discs 14 mm in diameter, cut with a biopsy punch, were placed upside down on agar (2.7%), one per well of a 24-well plate (Nalge Nunc International, Tokyo, Japan).
An adult female of F. occidentalis was introduced onto each leaf disc on a fine paintbrush. Each plate was covered with a micro-plate seal (UC-500; Ina Optica, Osaka, Japan), in which tiny holes for air circulation had been made with a fine injection needle, and placed in a climate-controlled room (25°C, 60% ± 10% RH, 16-h photoperiod). After 3 days, survivors and eggs were counted under a binocular microscope (MZ160 microscope with TL5000 Ergo light base with automatic aperture; Leica, Tokyo, Japan). We analysed 44 independent leaf discs from each transgenic line and WT plants.
Adult females of A. gossypii were similarly introduced. After 3 days' incubation as above, the survivors and nymphs were counted under the microscope. We analysed 40 independent leaf discs from each transgenic line and WT plants.
Adult females of T. ludeni were introduced onto leaf discs placed upside down on water-soaked cotton wool in a Petri dish (9 cm diameter, 1.7 cm deep). After 3 days' incubation as above, the survivors and eggs were counted under the microscope. We analysed 30 independent leaf discs from the transgenic lines and WT plants.
Author Contributions
AM, KM, TS, GA designed the study; AM, KM, TS, HK, RO, MN, GA, performed the experiments and data analyses; AM, KM, TS, GA wrote the manuscript; AI supervised the study.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Drs Koukichi Nagasaka, Shinya Tsuda and Yasuhiro Tomitaka for providing thrips and aphids; Dr. Hideto Miyoshi for HCAA analysis. This work was financially supported in part by Global COE Program A06 of Kyoto University; a research grant for Development and Assessment of Sustainable Humanosphere (DASH) system from the Research Institute for Sustainable Humanosphere, Kyoto University; and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to GA (No. 24770019) and TS (No. 23510271).
References
- von Röpenack E., Parr A. & Schulze-Lefert P. Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J. Biol. Chem. 273, 9013–9022 (1998). [DOI] [PubMed] [Google Scholar]
- Keller H. et al. Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum. Phytochemistry 42, 389–396 (1996). [Google Scholar]
- Muroi A. et al. Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana. Planta 230, 517–527 (2009). [DOI] [PubMed] [Google Scholar]
- Miyagawa H., Ishihara A., Nishimoto T., Ueno T. & Mayama S. Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp. avenae. Biosci. Biotechnol. Biochem. 59, 2305–2306 (1995). [Google Scholar]
- Mayama S., Matsuura Y., Iida H. & Tani T. The role of avenalumin in the resistance of oat to crown rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 20, 189–199 (1982). [Google Scholar]
- Mayama S., Tani T., Matauura Y., Ueno T. & Fukami H. The production of phytoalexins by oat in response to crown rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 19, 217–226 (1981). [Google Scholar]
- Schmidt A., Scheel D. & Strack D. Elicitor-stimulated biosynthesis of hydroxycinnamoyltyramines in cell suspension cultures of Solanum tuberosum. Planta 205, 51–55 (1998). [Google Scholar]
- Ishihara A. et al. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 54, 481–495 (2008). [DOI] [PubMed] [Google Scholar]
- Bernards M. A., Lopez M. L., Zajicek J. & Lewis N. G. Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J. Biol. Chem. 270, 7382–7386 (1995). [DOI] [PubMed] [Google Scholar]
- Yang Q., Reinhard K., Schiltz E. & Matern U. Characterization and heterologous expression of hydroxycinnamoyl/benzoyl-CoA:anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol. Biol. 35, 777–789 (1997). [DOI] [PubMed] [Google Scholar]
- Burhenne K., Kristensen B. K. & Rasmussen S. K. A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J. Biol. Chem. 278, 13919–13927 (2003). [DOI] [PubMed] [Google Scholar]
- D'Auria J. C. Acyltransferases in plants: a good time to be BAHD. Curr. Opin. Plant Biol. 9, 331–340 (2006). [DOI] [PubMed] [Google Scholar]
- Aida R. Torenia fournieri (torenia) as a model plant for transgenic studies. Plant Biotechnol. 25, 541–545 (2008). [Google Scholar]
- Wong W. S. et al. Study of cis-cinnamic acid in Arabidopsis thaliana. Plant Physiol. Biochem. 43, 929–937 (2005). [DOI] [PubMed] [Google Scholar]
- Holocomb G. E. First report of powdery mildew caused by an Oidium sp. on Torenia fournieri. Plant Disease 83, 878 (1999). [DOI] [PubMed] [Google Scholar]
- Stoessl A. Antifungal factors in barley. IV. Isolation, structure, and synthesis of the hordatines. Can. J. Chem. 45, 1745–1760 (1967). [Google Scholar]
- Aftab N., Likhitwitayawuid K. & Vieira A. Comparative antioxidant activities and synergism of resveratrol and oxyresveratrol. Nat. Prod. Res. 24, 1726–1733 (2010). [DOI] [PubMed] [Google Scholar]
- Okazaki Y. et al. Metabolism of avenanthramide phytoalexins in oats. Plant J. 39, 560–572 (2004). [DOI] [PubMed] [Google Scholar]
- Negrel J. & Jeandet P. Metabolism of tyramine and feruloyltyramine in TMV inoculated leaves of Nicotiana tabacum. Phytochemistry 26, 2185–2190 (1987). [Google Scholar]
- Zacarés L. et al. Induction of p-coumaroyldopamine and feruloyldopamine, two novel metabolites, in tomato by the bacterial pathogen Pseudomonas syringae. Mol. Plant Microbe Interact. 20, 1439–1448 (2007). [DOI] [PubMed] [Google Scholar]
- Cos P. et al. In vitro antioxidant profile of phenolic acid derivatives. Free Radical Res. 36, 711-716 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang H. L., Nagatsu A., Watanabe T., Sakakibara J. & Okuyama H. Antioxidative compounds isolated from safflower (Carthamus tinctorius L.) oil cake. Chem. Pharm. Bull. 45, 1910–1914 (1997). [DOI] [PubMed] [Google Scholar]
- An Y., Shen Y. & Zhang Z. Effects of mechanical damage and herbivore wounding on H2O2 metabolism and antioxidant enzyme activities in hybrid poplar leaves. J. For. Res. 20 (2009). [Google Scholar]
- Maffei M. E. et al. Effects of feeding Spodoptera littoralis on Lima Bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 140, 1022–1035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H. et al. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 53, 204–212 (2012). [DOI] [PubMed] [Google Scholar]
- Hegde M. et al. Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J. Chem. Ecol. 37, 741–750 (2011). [DOI] [PubMed] [Google Scholar]
- Suzuki K. et al. Flower color modifications of Torenia hybrida bycosuppression of anthocyanin biosynthesis genes. Mol. Breed. 6, 239–246 (2000). [Google Scholar]
- Aida R., Kishimoto S., Tanaka Y. & Shibata M. Modification of flower color in torenia (Torenia fournieri Lind.) by genetic transformation. Plant Sci. 153, 33–42 (2000). [Google Scholar]
- Shimoda T., Nishihara M., Ozawa R., Takabayashi J. & Arimura G. The effect of genetically enriched (E)-beta-ocimene and the role of floral scent in the attraction of the predatory mite Phytoseiulus persimilis to spider mite-induced volatile blends of torenia. New Phytol. 193, 1009–1021 (2012). [DOI] [PubMed] [Google Scholar]
- Mishiba K. et al. Strict de novo methylation of the 35S enhancer sequence in gentian. PLoS One 5, e9670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida R. & Shibata M. in Biotechnology in Agriculture and Forestry, Transgenic Crops III Vol. 48 (ed Y. P. S Bajaj) 294–305 (Springer Verlag., 2001).
- Murashige T. & Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962). [Google Scholar]
- Murai T. & Loomans A. J. M. Evaluation of an improved method for mass-rearing of thrips and a thrips parasitoid. Entomol. Exp. Appl. 101, 281–289 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information