Abstract
Background
Women with X-chromosome monosomy, or Turner syndrome (TS), are at increased risk for aortic dilation and dissection. To better understand the pathology and develop tools to monitor the risk of aortic disease, we investigated N-terminal pro brain natriuretic peptide (NT-proBNP) levels in women with TS and healthy female controls.
Methods
We evaluated NT-proBNP levels in women with karyotype-proven TS and healthy female volunteers in relation to ascending aortic diameter (AAD) and descending aortic diameter (DAD) measured by cardiovascular MRI.
Results
NT-proBNP levels were strongly and positively correlated with AAD and DAD in both cohorts. The TS group (n = 114, age = 37.4 ± 12 yr) had greater BSA-indexed aortic diameters and higher NT-proBNP levels than the control group (n = 27, age = 46.4 ± 11 yr): 88.3 ± 62.7 vs. 53.5 ± 35 pg/mL; P = 0.0003. Within the TS group, NT-proBNP levels were higher in those with dilated ascending aorta (n = 42; 112.4 ± 75.7 pg/mL) compared to those with normal aortic dimensions (n = 72; 74.2 ± 49 pg/mL, P = 0.0014). Abnormally high BNP levels were seen in 3 of 4 TS women that presented with previously undetected aortic aneurysm and/or dissection.
Conclusions
NT-proBNP levels are positively associated with aortic diameters in women with and without TS, suggesting a role for BNP in arterial wall homeostasis. Further study is necessary to determine whether NT-proBNP measurement may be used to monitor aortic diameter and/or detect aortic pathology in individuals at risk for aortic disease.
Introduction
Turner syndrome (TS) is a relatively common chromosomal disorder affecting approximately 1/2000 live female births1. It is caused by the complete or partial loss of a second sex chromosome during embryonic development, with or without cell line mosaicism. Nearly all individuals have short stature and premature ovarian failure, and approximately 50% have congenital cardiovascular defects2. Indeed, congenital cardiovascular disease with an increased risk for aortic dilation and dissection is the most medically significant feature of TS and is the major cause of premature mortality in these women3–5. Identification and monitoring of individuals at risk for acute aortic events is clearly of great importance. Due to the small stature of women with TS, aortic diameters must be evaluated in relation to body surface area by use of aortic size index (ASI) 4.
Brain natriuretic peptide (BNP) is a cardiac hormone with natriuretic, diuretic, and vasorelaxant properties6. It is secreted by ventricular myocytes in response to volume expansion and pressure overload, and plasma concentrations are elevated in patients with congestive heart failure and ventricular hypertrophy6–7. In addition to functioning as a circulating hormone involved in body fluid homeostasis8 as well as an autocrine and/or paracrine factor involved in cardiac remodeling9–12, BNP plays a role in vascular regulation. The natriuretic peptide A receptor (NPR-A) that binds BNP is present in the vascular wall13, and the biological importance of BNP in the control of vascular function has been demonstrated in recent studies. For example, natriuretic peptide receptor blockade increases coronary vascular resistance14 and genetic disruption of the NPR-A receptor results in hypertension, left ventricular hypertrophy and sudden death15. Schirger, et al. demonstrated that BNP inhibits human aortic vascular smooth muscle cell proliferation and potently relaxes the normal rabbit aorta16. Furthermore, Sbarouni et al. found that levels of the precursor N-terminal proBNP (NT-proBNP) were significantly higher in patients with acute aortic dissection and chronic aortic aneurysm compared to normal patients17. Because of the greater stability and longer half-life of NT-proBNP compared to BNP, the former is predominantly measured in clinical studies.
In the present study we aimed to determine if circulating NT-proBNP could be a biomarker related to aortic diameters in women at risk for aortic dilation because of TS. Thus we compared NT-proBNP levels in a large cohort with TS and a group of healthy women all of whom had aortic diameters and cardiac function measured by cardiac magnetic resonance imaging.
Methods
Study Subjects
Study subjects were part of an ongoing National Institute of Child Health and Human Development Institute Review Board-approved TS natural history protocol (00-CH-0219; NCT 00006334). Subjects were recruited through notices on the NIH (http://turners.nichd.nih.gov) and Turner Syndrome Society websites (www.turner-syndrome.org). Healthy female volunteers were recruited through the NIH office of normal volunteers and all participants gave written informed consent. The diagnosis of TS was confirmed by 50-cell peripheral karyotype in which 70% or more of cells demonstrated loss of all or part of the second sex chromosome.
Participants in this study included 114 adults with TS enrolled from 2008–2011, and 27 healthy adult female volunteers who were studied during the same time period. All study participants had normal cardiovascular and renal function. TS participants with recent cardiac or aortic surgery (n = 7) or a left ventricular ejection fraction of less than 50% (n = 3) were excluded. Women with creatinine clearance corrected for BSA less than 60 mL/min/m2 (n = 3) were also excluded. Height and weight for each participant were measured by NIH Clinical Research Center nurses using a SRScale, model SR555, with a height rod. Body surface area was calculated with the DuBois formula.
NT-proBNP Measurement
Venous blood was collected in plasma separator tubes at the NIH Clinical Center and centrifuged. Plasma was then removed and NT-proBNP was measured by immunoassay at the NIH Department of Laboratory Medicine using a Siemens Dimension Vista® System Analyzer, within 4 hours of specimen collection. This assay is described in detail on the NIH Clinical Center Lab Directory website (http://cclnprod.cc.nih.gov/dlm/testguide).
Magnetic Resonance Imaging
Magnetic resonance imaging techniques used in our study of the thoracic aorta included cine MRI, black blood imaging, three-dimensional MRA with and without contrast and velocity-encoded cine phase contrast pulse sequences. Cross-sectional measurements of the ascending and descending thoracic aorta were performed on axial T-1 weighted black blood images at the level of the pulmonary artery bifurcation, perpendicular to the long axis of the ascending aorta. Left ventricular volumes and ejection fraction were determined using steady-state free precession cine imaging as previously described18.
Statistical Analysis
Data are expressed as means ± standard deviation or percentage. To approximate normal distribution, NT-proBNP values were log transformed for regression analyses. For more clinical relevance, absolute or age-specific abnormal NT-proBNP values are compared in TS vs. control groups. The group means were compared by ANOVA or ANCOVA with age and BSA as the covariates where indicated. Simple and multiple regression analyses were used to relate NT-proBNP levels to age, BMI and aortic diameters. Statistical significance was set at values of P < 0.05. All analyses were performed on Stat View version 5.0.1 (SAS Institute Inc., Cary, NC).
No extramural funding was used to support this work
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents
Results
TS participants ranged in age from 18 to 67 years (37.4 ± 12.3), and healthy female volunteers from 26 to 65 (46.4 ± 11.4 yr). Both groups were approximately 90% Caucasian with the remainder approximately equally composed of African American, Hispanic and Asian individuals. Renal function was normal in all participants determined by normal creatinine and blood urea nitrogen tests.
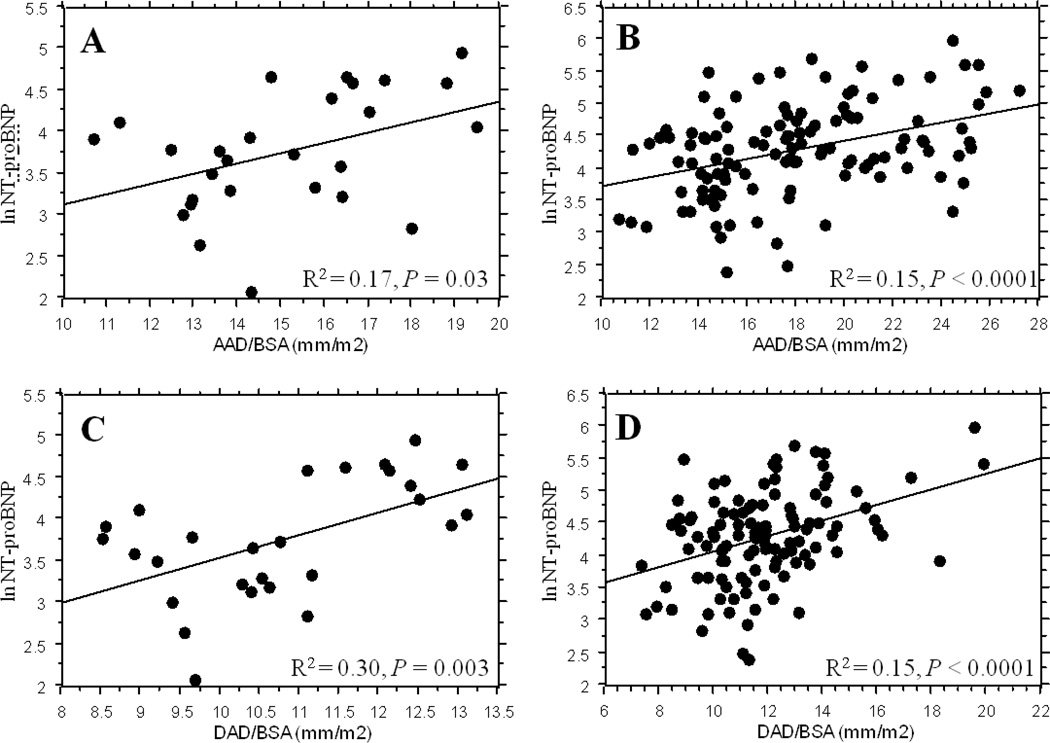
NT-proBNP levels reflect ascending and descending aortic diameters
Simple regression analysis shows a highly significant correlation between plasma NT-proBNP level and both ascending and descending aortic diameters (indexed to body size) in women with TS and in healthy volunteers (Figure 1). There was no significant correlation between NT-proBNP levels and, The average systolic blood pressure (r = 0.121, P = 0.20), diastolic blood pressures (not shown), left ventricular ejection fraction (r = 0.051, P = 0.59), left ventricular end diastolic volume (r = 0.022, P = 0.82), anteroseptal index (r = 0.16, P = 0.10), or serum creatinine (r = 0.03, P = 0.77) did not correlate with NT-proBNP levels and were similar in the two groups. However, NT-proBNP levels were positively correlated with age (r = 0.27, P < 0.01), and negatively correlated with BMI (r = −0.19, P = 0.034). In multiple a regression analysis with BMI, age and AAD/BSA as independent variables, only AAD/BSA remained a significant factor predicting NT-proBNP level (P < 0.0001). Likewise, with BMI, age and DAD/BSA as independents, only DAD/BSA was significant for NT-proBNP level (P < 0.0001).
Figure 1.
Correlation between ascending and descending aortic diameters (indexed to body size) and NT-proBNP in healthy female controls (A and C) and women with TS (B and D). The difference in the axis scales reflects the wider range of aortic diameters in the TS group.
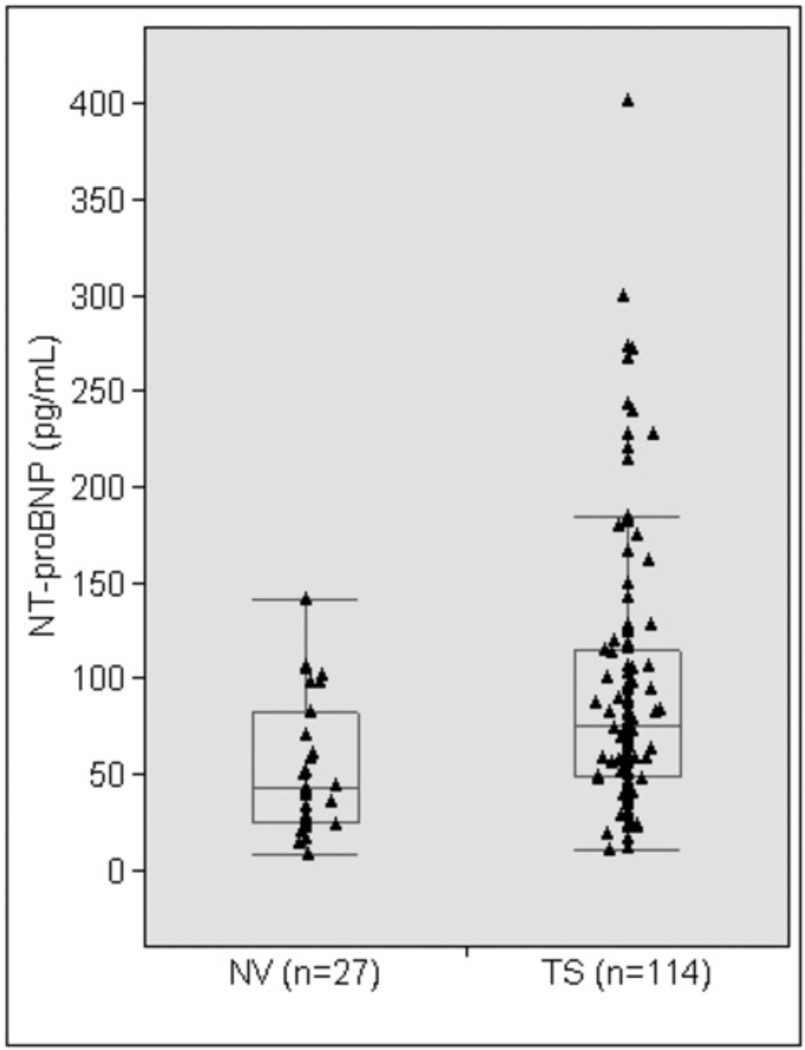
NT-proBNP levels in TS and in Healthy Controls
Characteristics of TS study participants and healthy volunteers are shown in Table I. The TS group was younger and smaller in height and BSA. Average systolic and diastolic blood pressures were similar in the two groups. Since age is a significant factor in aortic diameters and NT-proBNP levels, age was used as a covariate in analyses comparing these outcomes. Though mean absolute ascending and descending aortic diameters were similar in the two groups, when indexed to BSA both ascending and descending ASI were significantly greater in the TS group. Mean NT-proBNP levels were significantly greater in the TS group compared to the healthy volunteers (Table I; Figure 2). After excluding TS participants with a dilated aorta, mean NT-proBNP levels remained significantly greater in the TS group (74.2 ± 49.0 pg/mL; n = 72) compared to the control group (53.5 ± 35.0 pg/mL; n = 27), P = 0.036.
Table I.
Aortic Diameters and NT-proBNP in TS and Healthy Female Volunteers
| TS (n = 114) | NV (n = 27) | P | |
|---|---|---|---|
| Age, y | 37.4 ± 12.3 | 46.4 ± 11.4 | <0.001 |
| Height, cm | 147.6 ±7.3 | 162.6 ± 6.0 | <0.001 |
| BSA, m2 | 1.56 ± 0.21 | 1.73 ± 0.17 | <0.001 |
| Systolic BP, mm Hg | 120.6 ± 12.0 | 121.1 ± 14.6 | 0.849* |
| AAD, mm | 27.4 ± 5.1 | 25.9 ± 4.1 | 0.129* |
| AAD/BSA mm/m2 | 17.9 ± 3.9 | 15.1 ± 2.4 | <0.001* |
| DAD, mm | 18.1 ± 2.9 | 18.5 ± 2.6 | 0.533* |
| DAD/BSA mm/m2 | 11.8 ± 2.3 | 10.8 ± 1.4 | 0.009* |
| NT-proBNP, pg/mL | 88.3 ± 62.7 | 53.5 ± 35.0 | 0.0003* |
Values are means ± SD. Mean values compared by ANOVA or *ANCOVA with age as a covariate. NV, normal volunteer; AAD & DAD, ascending and descending aortic diameters.
Figure 2.
Circulating NT-proBNP levels in TS women compared to healthy female controls. The box includes the 25th to 75th percentiles and the whiskers include the 10th to 90th percentiles. The horizontal line in the box indicates the median.
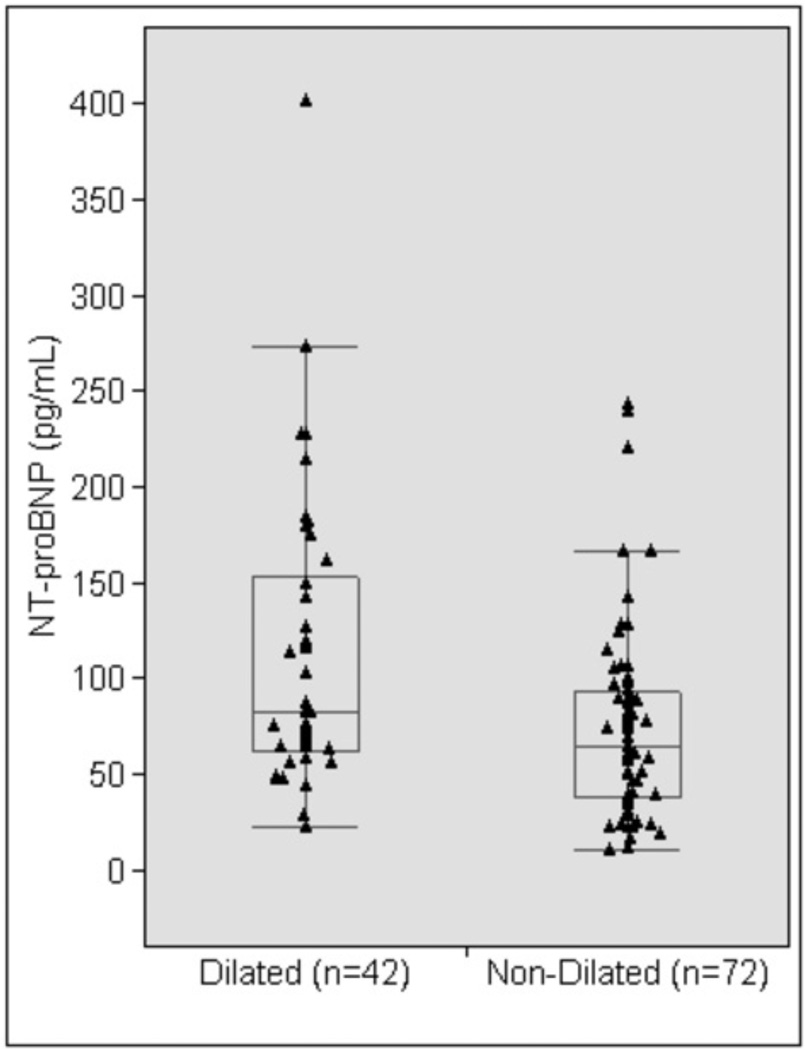
NT-proBNP levels reflect aortic dilation in TS
NT-proBNP levels for TS study participants stratified by aortic dilation are shown in Table II and Figure 3. Aortic dilation was defined as an AAD/BSA (ASI) > 19.6 mm/m2, exceeding the 95th percentile for age-matched control women4. Controlling for differences in age and BSA, mean NT-proBNP levels were significantly greater in the dilated group compared to the non-dilated group (Table II; Figure 3). When BMI was used as a covariate in addition to age, NT-proBNP levels remained significantly greater in the dilated group (P = 0.0014). Individual patient NT-proBNP level was above the upper limit of normal for age19 in only 9 of the 42 (21.4%) women with dilated aorta. All of the healthy female volunteers had NT-proBNP levels within the normal range.
Table II.
NT-proBNP Levels in TS Study Participants Stratified by Aortic Dilation
| Dilated‡ Ascending Aorta (n = 42) |
Non-Dilated Ascending Aorta (n = 72) |
P | |
|---|---|---|---|
| Age, y | 44.6 ± 12.0 | 33.1 ± 10.5 | < 0.001 |
| BSA, m2 | 1.44 ± 0.18 | 1.62 ± 0.19 | < 0.001 |
| Systolic BP, mm Hg | 121.4 ± 14.5 | 120.2 ± 10.3 | 0.582* |
| AAD, mm | 31.7 ± 4.5 | 24.9 ± 3.4 | < 0.001* |
| AAD/BSA mm/m2 | 22.2 ± 2.3 | 15.4 ± 2.0 | < 0.001* |
| DAD, mm | 19.2 ± 2.9 | 17.6 ± 2.7 | 0.003* |
| DAD/BSA mm/m2 | 13.4 ± 2.2 | 10.9 ± 1.8 | < 0.001* |
| NT-proBNP, pg/mL | 112.4 ± 75.7 | 74.2 ± 49.0 | 0.0014† |
Values are means ± SD. Mean values compared by ANOVA or *ANCOVA with age as a covariate or †ANCOVA with age and BSA as covariates.
Dilated ascending aorta: AAD/BSA ≥ 19.6 mm/m2.
Figure 3.
Circulating NT-proBNP levels in TS women with a dilated ascending aorta compared to TS women with a non-dilated ascending aorta. The box includes the 25th to 75th percentiles and the whiskers include the 10th to 90th percentiles. The horizontal line in the box indicates the median.
Aortic pathology and NT-proBNP in TS
Four women presented with previously undiagnosed aortic pathology during the course of this study from 2008–2011. Three (two with aortic dissection and one with ascending aortic aneurysm) had NT-proBNP levels well above the upper limit of normal for age-matched normal women. One woman with aortic aneurysm and morbid obesity had a normal NT-proBNP level.
Discussion
In this study, we set out to determine whether measurement of circulating NT-proBNP levels could discriminate the subset of individuals with Turner syndrome with significant aortic dilation. We found a remarkable linear correlation between both ascending and descending aortic diameters and NT-proBNP values in women with TS. To determine if this effect was limited to a “Turner aortopathy”, we also measured the peptide in our cohort of healthy females participating in aortic evaluation by MRI. These novel data show that NT-proBNP levels are positively correlated with ascending and descending aortic diameters in healthy volunteer women as well as women with TS. Most of the TS participants were normotensive and in good general health, and all had normal left ventricular size and function demonstrated by MRI. Certainly the normal volunteers were in good health and also had normal left ventricular size and function determined by MRI. Thus, the positive correlation between NT-proBNP and aortic diameters documented in this study suggests a potential physiologic role for BNP in aortic wall homeostasis. This possibility is reinforced by our finding a highly significant correlation between NT-proBNP levels and descending aortic diameter, which is relatively dissociated from direct cardiac effects compared to the ascending aorta. Moreover, there is abundant evidence that BNP exerts direct effects on vascular smooth muscle cells from human and rabbit aorta16 and recent data suggest it may also be produced by the arterial wall20.
Large population-based studies show a high degree of inter-subject variability in NT-proBNP values even in healthy women. Normative data show that age and diastolic BP are positive and BMI and diastolic BP negative correlates of NT-proBNP levels19. Consistent with these data, we found a significant positive correlation with age, and negative relation to BMI that bordered on significant. Our study subjects were relatively young and normotensive compared to previous studies, and most had NT-proBNP levels within the normal range. In fact, the average value of 53.5 pg/ml for our NV group matches the 50th quantile value for women in this age group19. The TS group average NT-proBNP value of 88.3 pg/ml is two-fold higher than the 50th quantile for their age group19, but still within the normal range. These observations support a relevant physiological connection between circulating NT-proBNP level and aortic diameter. Our results on the correlation between aortic diameters and NT-proBNP levels are not likely explained by variations in renal clearance among our study participants. All subjects were judged to have normal renal function based on medical history, estimated GFR and creatinine clearance. Moreover, we found no relation between serum creatinine levels or creatinine clearance and NT-proBNP levels in our study subjects.
The significantly higher average NT-proBNP levels in women with TS compared to healthy female controls is consistent with an inherent aortopathy in the syndrome reflected by the relatively larger sizes of the ascending and descending aorta. Because the TS group was younger than controls, the difference in NT-proBNP levels may actually be underestimated since NT-proBNP levels generally increase with age. Moreover, NT-proBNP levels did reflect aortic dilation in our TS group. The women with BSA-indexed ascending aortic dilation had significantly greater NT-proBNP levels compared to women with TS with normal aortic dimensions and compared to healthy controls. However, the NT-proBNP levels of TS women with significant dilation of the ascending aorta were above the upper limit of age group-specified normal in only 9/42 or 21% of cases. This upper limit criteria is not so sensitive given the very wide population-based reference limits19. Given the significantly lower intra-subject variation for NT-proBNP values19, it is possible that longitudinal tracking of NT-proBNP in individual patients will be more helpful in identifying aortic disease. Importantly, three of the four women that presented to the study with critical yet unsuspected aortic pathology had NT-proBNP levels well above their age-range specific upper limits. Additional studies are necessary to validate the usefulness of NT-proBNP measurements for monitoring aortic dilation in TS patients, with elevated levels indicating need for cardiovascular MR or other imaging.
We found only one previous study that investigated NT-proBNP levels in relation to aortic pathology17. This prior study compared D-dimers and NT-proBNP levels in 18 patients with acute aortic dissection, 21 patients with chronic aortic aneurysm and 8 controls. The participants were predominantly male, older and hypertensive with undefined left ventricular status, and thus not very similar to our study subjects. However, NT-proBNP levels were quite elevated in both the aortic dissection and chronic aneurysm groups compared to the small control group17. Although these data are suggestive, these study subjects seem to have very compromised cardiovascular status and the relation of NT-proBNP levels to pathologic aortic parameters as opposed to left ventricular strain and/or failure is unknown.
The major limitations of this study are our small sample size for aortic pathology and the lack of serial measurements, so we are unable to predict the usefulness of NT-proBNP measurements in diagnosing or monitoring aortic disease in TS. However, the data demonstrating significant correlation between ascending and descending aortic diameters in TS and in healthy volunteers is both novel and robust, supporting the importance of further investigations into the potential role of BNP in aortic physiology and pathology.
Acknowledgments
This work was supported by the intramural research program of the NIH. No extramural funding was used to support this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser. 1990;26(4):209–223. [PubMed] [Google Scholar]
- 2.Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004 Sep 21;110(12):1694–1700. doi: 10.1161/01.CIR.0000142290.35842.B0. [DOI] [PubMed] [Google Scholar]
- 3.Gravholt CH, Landin-Wilhelmsen K, Stochholm K, et al. Clinical and epidemiological description of aortic dissection in Turner's syndrome. Cardiol Young. 2006 Oct;16(5):430–436. doi: 10.1017/S1047951106000928. [DOI] [PubMed] [Google Scholar]
- 4.Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007 Oct 9;116(15):1663–1670. doi: 10.1161/CIRCULATIONAHA.106.685487. [DOI] [PubMed] [Google Scholar]
- 5.Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007 Dec;44(12):745–749. doi: 10.1136/jmg.2007.052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998 Jul 30;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 7.Cowie MR, Mendez GF. BNP and congestive heart failure. Prog Cardiovasc Dis. 2002 Jan-Feb;44(4):293–321. doi: 10.1053/pcad.2002.24599. [DOI] [PubMed] [Google Scholar]
- 8.Luchner A, Stevens TL, Borgeson DD, et al. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol. 1998 May;274(5 Pt 2):H1684–H1689. doi: 10.1152/ajpheart.1998.274.5.H1684. [DOI] [PubMed] [Google Scholar]
- 9.Holtwick R, van Eickels M, Skryabin BV, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003 May;111(9):1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoun AM, Liang F, O'Young G, et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004 Mar 5;94(4):453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 11.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuruda T, Boerrigter G, Huntley BK, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002 Dec 13;91(12):1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 13.Suga S, Nakao K, Hosoda K, et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992 Jan;130(1):229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 14.Supaporn T, Wennberg PW, Wei CM, Kinoshita M, Matsuda Y, Burnett JC. Role for the endogenous natriuretic peptide system in the control of basal coronary vascular tone in dogs. Clin Sci (Lond) 1996 May;90(5):357–362. doi: 10.1042/cs0900357. [DOI] [PubMed] [Google Scholar]
- 15.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995 Nov 2;378(6552):65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 16.Schirger JA, Grantham JA, Kullo IJ, et al. Vascular actions of brain natriuretic peptide: modulation by atherosclerosis and neutral endopeptidase inhibition. J Am Coll Cardiol. 2000 Mar 1;35(3):796–801. doi: 10.1016/s0735-1097(99)00593-8. [DOI] [PubMed] [Google Scholar]
- 17.Sbarouni E, Georgiadou P, Marathias A, Geroulanos S, Kremastinos DT. D-dimer and BNP levels in acute aortic dissection. Int J Cardiol. 2007 Nov 15;122(2):170–172. doi: 10.1016/j.ijcard.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 18.Aletras AH, Tilak GS, Hsu L-Y, Arai AE. Heterogeneity of Intramural Function in Hypertrophic Cardiomyopathy / Clinical Perspective. Circulation: Cardiovascular Imaging. 2011 Jul 1;4(4):425–434. doi: 10.1161/CIRCIMAGING.110.958751. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study) Am J Cardiol. 2011 Nov 1;108(9):1341–1345. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KR, Hoagland TM, Olson KR. Endogenous vascular synthesis of B-type and C-type natriuretic peptides in the rainbow trout. J Exp Biol. 2011 Aug 15;214(Pt 16):2709–2717. doi: 10.1242/jeb.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]