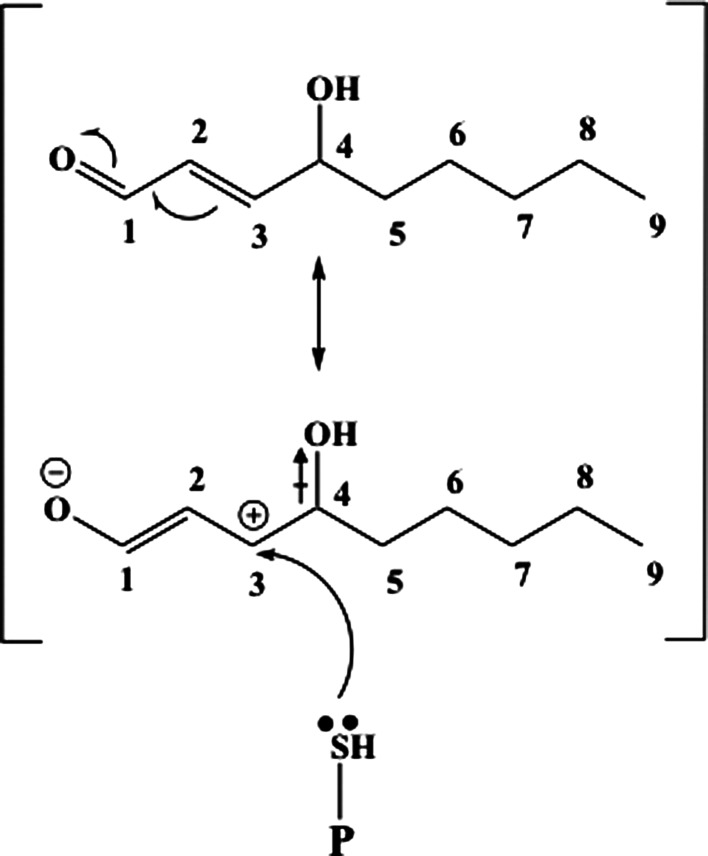
FIG. 4.
The first step of Michael addition. C-3 has electron density moved toward O atom of OH on C-4 due to inductive effect ( ). At the same time, resonance between the double bond between C-3 and C-2 and the aldehyde dumps electron density away from C-3. The net effect is a partially positive C-3 that becomes a target for electrophilic reaction of lone pair of electrons on the S atom of cysteine.
). At the same time, resonance between the double bond between C-3 and C-2 and the aldehyde dumps electron density away from C-3. The net effect is a partially positive C-3 that becomes a target for electrophilic reaction of lone pair of electrons on the S atom of cysteine.