Abstract
Purpose.
To assess the prevalence of peripheral fundus autofluorescence (FAF) abnormalities in a variety of diseases seen at a tertiary retina clinic.
Methods.
We conducted a retrospective review of cases seen at the Doheny Eye Institute between November 2009 and May 2011, who had ultra-widefield FAF and pseudocolor imaging performed on new models of scanning laser ophthalmoscopes. Patients with a history of previous therapies that could alter the FAF findings, including vitrectomy, cryotherapy, laser photocoagulation, or photodynamic therapy, were excluded from the analysis. Based on their primary diagnosis the eyes were grouped into nine disease categories: age-related macular degeneration, central serous retinopathy, dystrophy, inflammatory disorders, ocular tumor, retinal vascular disorders, other, normal, and unknown. All FAF and accompanying pseudocolor images were reviewed independently by two reading center–certified graders.
Results.
A total of 470 eyes of 248 patients were included for analysis of which 461 eyes had images of sufficient quality for grading. The prevalence of peripheral findings was 65.5% (n = 302) for FAF images and 68.5% (n = 316) for the pseudocolor images (P < 0.001). The prevalence of peripheral abnormalities differed significantly between the disease categories ranging from 18.5% to 82.2% for FAF and 18.5% to 82.4% for pseudocolor images.
Conclusions.
Peripheral FAF abnormalities are frequent and readily revealed by FAF imaging. Interestingly, even cases with presumably macular disease demonstrated a high prevalence of peripheral findings. Further investigation in prospective studies is warranted.
Peripheral abnormalities on ultra-widefield autofluorescence images are common in a large variety of diseases and reliably identifiable. There is a high correspondence between changes seen on autofluorescence and pseudocolor images. Peripheral changes in ultra-widefield imaging are a common finding in a variety of different diseases.
Introduction
Abnormalities in the peripheral fundus in vivo have been frequently described since the introduction of the ophthalmoscope by von Helmholtz in 1851. Among the reported peripheral findings are pigmentary changes, patches of retinal atrophy, retinal tears, proliferative vascular complexes, and choroidal tumors, to name only a few. The clinical significance of these findings varies widely from incidental findings with no visual consequence, to vision-threatening abnormalities if left untreated.1–4 In this context, noninvasive imaging technologies, in particular color photographic approaches, have long played a secondary role in clinical practice as their function was reduced to the objective documentation of pathologies that were primarily identified through fundoscopy. The advent of fundus autofluorescence (FAF) imaging justifies a reinterpretation of this role. FAF is based on the principle that fluorophores are excited by light of a certain wavelength and, in turn, emit a characteristic light spectrum.5 The images provided by FAF contain information that is not visible or poorly visible via conventional noninvasive methods alone yet valuable for clinicians in their day-to-day clinical practice. It may aid in the early diagnosis of diseases such as idiopathic macular telangiectasia type II6 or hydroxychloroquine toxicity,7 and provides a tool for monitoring the evolution of geographic atrophy secondary to age-related macular degeneration (AMD). The prognostic value of certain FAF patterns for predicting the rate of progression of geographic atrophy was identified by Holz et al.8 and is important for both clinical trials and clinical practice, and underscores the utility and benefits of FAF imaging.
On the downside, FAF image acquisition requires specialized equipment,9 and much of its clinical research is limited by the technical specifications of this equipment. So far only few devices are available for FAF imaging. The scanning laser ophthalmoscope (HRA-2; Heidelberg Engineering, Heidelberg, Germany) and the modified fundus camera (Topcon, Tokyo, Japan) equipped with bandwidth filters developed by Spaide et al.10 are the most widely used devices to date. Accordingly, the vast majority of scientific publications on FAF are based on these two systems.11
Although both cameras provide FAF images of excellent quality, they have a limited field of view (FOV, 30 to 50°), and most imaging research to date has consequently focused on the posterior pole. Although it is possible to construct montages of several adjacent FOVs and thus enable a larger field and more peripheral evaluation, this is a time-consuming process, and the far periphery is usually still not demonstrated by this approach. Furthermore, abnormalities occurring at the boundaries of the overlapping images may be obscured by, or mistaken for, artifacts. These limitations spawned the development of a variety of widefield imaging devices (RetCam; Clarity Medical Systems, Pleasanton, CA; Panoret; Medibell Medical Vision Technologies, Haifa, Israel; Optomap; Optos, Dunfermline, Scotland, UK). This last scanning laser ophthalmoscope (SLO) device in particular was able to demonstrate acquisition of single-frame images with a noncontact approach, with an FOV of >150°.12,13
Recently, the optomap device was updated to include greenlight autofluorescence capability in addition to the pseudocolor SLO images, allowing far peripheral FAF abnormalities to be evaluated for the first time. We used this approach to study the prevalence and patterns of peripheral abnormalities visible on widefield FAF and pseudocolor imaging in a retinal disease patient population at a tertiary care academic medical center.
Materials and Methods
In a retrospective review, we evaluated all cases that had undergone ultra-widefield imaging with the optomap system at the Doheny Eye Institute over an 18-month period between November 2009 and May 2011. This study was approved by the Institutional Review Board of the University of Southern California and adhered to the tenets set forth in the Declaration of Helsinki. Initial imaging was done with an ultra-widefield SLO (P200CAF; Optos), a prototype device for clinical testing. In September of 2010, this device was replaced with another commercially available model (P200Tx) that offers the same functionality. This ultra-widefield SLO can acquire autofluorescence images based on an excitation wavelength of 532 nm. In addition, it is capable of capturing pseudocolor images, which are composed of red and green reflectance images but without the blue color channel. The FOV for single-frame fundus images using this technique exceeds 150°, with claims that some images may approach 200°.
Since an objective of our study was to compare the frequency of abnormalities seen on pseudocolor versus FAF images, for the purpose of this analysis, only cases with both FAF images and pseudocolor images were included in the final evaluation. The pseudocolor images and corresponding FAF images for a given case were taken by the same photographer only minutes apart, thus reducing possible bias introduced through different device operators or different acquisition times of the two modalities. Eyes that had been treated with peripheral laser, photodynamic therapy, cryotherapy, or eyes that had undergone a surgical intervention were also excluded because these treatments would potentially create pseudocolor and FAF abnormalities unrelated to the disease process itself.
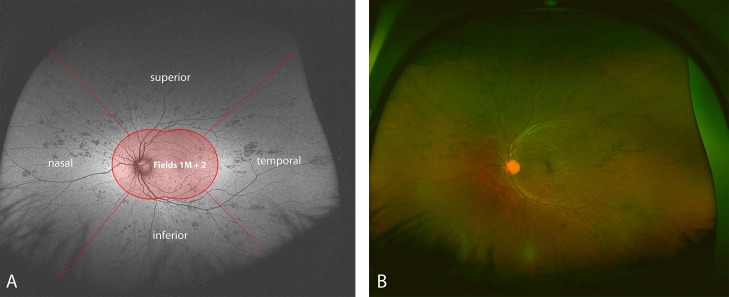
All grading was performed by two independent, masked, Doheny Image Reading Center (DIRC)–certified graders (FMH, CST). The images were viewed in the proprietary review software (V2 Vantage Dx, version 2.5.0.135; Optos) and individually adjusted in contrast and brightness for optimal visualization of retinal features. The quality and usability of each image were assessed subjectively by each grader. Autofluorescence images and pseudocolor images were graded separately for the presence of peripheral abnormalities outside the central FOV (Fig. 1). This central FOV was defined as a combination of the standard field 2 and field 1M photographs from the modified 7-standard fields used in the Age-Related Eye Disease Study (AREDS). FAF abnormalities were defined as changes from the background autofluorescence, manifesting as relative hyper- or hypofluorescence. Pseudocolor abnormalities were defined as any changes from normal fundus appearance, based on the central grading protocol of the DIRC. Uniform changes in choroidal pigmentation or generally different pigmentation levels across the whole fundus were therefore not graded as abnormalities, as opposed to focal pigmentation changes, hemorrhages, or vascular abnormalities. Peripheral changes were graded for their presence (yes/no), as well as for their regional location by quadrant (superior, inferior, temporal, or nasal; Fig. 1). Abnormalities could space one or many quadrants. The area or extent of the abnormality was not quantified in this series. The grading was performed using a standard reading center dual-grader process, where each case was independently fully graded by both graders. Pseudocolor and FAF images were graded nonsimultaneously in a masked fashion. To arrive at a single final determination for each question for each case, all discrepant answers were resolved by open adjudication in which both graders reevaluated the case together. For cases in which the two graders could not come to an agreement, a third senior grader (SRS) cast the deciding vote. However, for these discrepant cases, the original grading of each grader was retained in the database for subsequent intergrader agreement analysis.
Figure 1. .
FAF (A) and corresponding pseudocolor image (B) of a case with atypical acute multifocal placoid pigment epitheliopathy. The FAF image is schematically sectioned to illustrate the grading process. Abnormalities outside the central FOV (labeled as Fields 1M + 2) were graded according to their location in either the superior, temporal, inferior, or nasal quadrant. Of note, the peripheral changes correlate well between FAF and pseudocolor in this case.
Cases were binned into groups according to their primary diagnosis as determined by each patient's referring physician, and statistical analysis was performed with a commercially available statistics software program (Stata, v. 10.1; StataCorp LP, College Station, TX). The χ2 test was used to test for significant differences between various groups. The level of significance was defined as P < 0.05 for all analyses.
This study fully adhered to the principles set forth in the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Southern California.
Results
A total of 411 patients and 627 eyes were retrospectively reviewed. Of these cases, 23 eyes were excluded due to iatrogenic peripheral lesions, 137 eyes were excluded because only pseudocolor images were obtained, 6 eyes were excluded because only FAF images were obtained, and 9 eyes were excluded because either of the FAF pseudocolor images were deemed to be of insufficient quality for grading. The final analysis included 461 eyes of 247 patients with both FAF images and pseudocolor images. The mean age was 59.6 years (SD ±20.1 years), and 56.4% were female. Based on their primary diagnosis made by the treating physician the eyes were grouped into 9 disease categories: AMD (n = 135), central serous retinopathy (CSR; n = 17), retinal dystrophy (n = 91), inflammatory disorders (n = 108), ocular tumors (n = 11), retinal vascular disease (n = 18), other (n = 48), normal (n = 26), and unknown (i.e., treating physician was not able to make a definitive diagnosis; n = 16). The retinal vascular disease category included eyes with diabetic retinopathy and vascular occlusions, whereas eyes grouped as “other” had a variety of miscellaneous primary diagnoses including cataract, primary open-angle glaucoma, or posterior vitreous detachment (Table 1).
Table 1. .
Diagnoses Grouped Together as “Other”
|
Primary Diagnosis |
Frequency (n) |
| Myopia | 10 |
| Posterior vitreous detachment | 8 |
| Peripheral drusen | 7 |
| Peripheral degeneration | 6 |
| Choroidal nevus | 4 |
| Retinal detachment | 3 |
| Polypoidal choroidal vasculopathy | 2 |
| Primary open-angle glaucoma | 2 |
| CHRPE | 1 |
| Epiretinal membrane | 1 |
| Hypotony maculopathy | 1 |
| Pigmentary retinopathy | 1 |
| Plaquenil toxicity | 1 |
| Teleangiectasia type 2 | 1 |
CHRPE, congenital hyperplasia of the retinal pigment epithelium.
Adjudicated Results
The prevalence of peripheral findings differed slightly between FAF images (n = 302) and the pseudocolor images (n = 316). A χ2 test revealed that this difference was statistically significant (P < 0.001). Also, the prevalence of peripheral abnormalities differed significantly between the disease groups for both FAF (P < 0.001) and pseudocolor images (P < 0.001). Interestingly, even eyes classified as “AMD” or “CSR” showed a noteworthy prevalence of peripheral FAF changes ranging from 52.6% to 73.9% and high concordance with pseudocolor grading. General characteristics of the groups and the prevalence of peripheral abnormalities per disease category are summarized in Table 2.
Table 2. .
Summary of the Frequency of Peripheral Findings
|
Disease Category |
Frequency of Peripheral Findings |
||||
|
Age (y) (SD) |
Female (%) |
FAF, n (%) |
Color, n (%) |
Ungradable Eyes, n (%) |
|
| AMD (n = 135) | 79.7 (9.1) | 55.6 | 73.9 | 79.3 | 1 (0.7) |
| CSR (n = 17) | 50.4 (9.0) | 21.1 | 52.6 | 52.9 | 0 |
| Dystrophy (n = 91) | 49.4 (18.5) | 60.9 | 82.2 | 82.4 | 1 (1.1) |
| Inflammatory (n = 108) | 48.8 (17.1) | 70.5 | 68.5 | 71.4 | 4 (3.7) |
| Ocular tumor (n = 11) | 55.4 (22.0) | 45.5 | 72.7 | 81.8 | 0 |
| Vascular (n = 18) | 44.9 (17.3) | 22.2 | 47.1 | 52.9 | 1 (5.6) |
| Other (n = 48) | 58.1 (17.1) | 51 | 42.2 | 44.4 | 1 (2.1) |
| Normal* (n = 26) | 53.9 (19.6) | 51.9 | 18.5 | 18.5 | 1 (3.8) |
| Unknown (n = 16) | 51.7 (13.9) | 47.1 | 56.3 | 56.3 | 0 |
Normal = no pathologies in the posterior chamber were noted during clinical examination.
Grading of the FAF images revealed that the superior quadrant was involved in 44.7% of eyes, the inferior quadrant in 45.8%, the temporal quadrant in 51%, and the nasal quadrant in 54%. A total of 163 eyes (35.3%) showed peripheral abnormalities in all four quadrants. Similarly, the pseudocolor grading revealed a regional distribution of changes for superior (51.4%), inferior (49%), temporal (54.7%), and nasal (57.9%) quadrants. There was no apparent significant difference or trend observable in the localization of the peripheral changes by disease group (Table 3).
Table 3. .
Summary of the Frequency per Quadrant
|
Disease Category |
Frequency of Peripheral Findings in Percent |
|||
|
Upper Quadrant Pseudocolor (FAF) |
Lower Quadrant Pseudocolor (FAF) |
Nasal Quadrant Pseudocolor (FAF) |
Temporal Quadrant Pseudocolor (FAF) |
|
| AMD (n = 135) | 60.7 (43.3) | 54.8 (48.5) | 71.1 (63.4) | 62.2 (53.0) |
| CSR (n = 17) | 26.4 (31.6) | 5.9 (26.3) | 17.7 (26.3) | 23.5 (21.1) |
| Dystrophy (n = 91) | 72.5 (71.1) | 73.6 (70.0) | 75.8 (75.6) | 75.8 (76.7) |
| Inflammatory (n = 108) | 50.5 (50.0) | 50.5 (51.9) | 57.9 (54.6) | 59.8 (57.4) |
| Ocular tumor (n = 11) | 36.4 (27.3) | 27.3 (27.3) | 45.5 (45.5) | 45.5 (45.5) |
| Vascular (n = 18) | 41.2 (35.3) | 29.4 (29.4) | 52.9 (35.3) | 29.4 (29.4) |
| Other (n = 48) | 25.5 (17.0) | 29.8 (14.9) | 31.9 (25.5) | 27.7 (21.3) |
| Normal* (n = 26) | 11.5 (11.5) | 15.4 (11.5) | 11.5 (15.4) | 15.4 (15.4) |
| Unknown (n = 16) | 50.0 (36.4) | 43.8 (43.8) | 50.0 (50.0) | 63.6 (50.0) |
Bold numbers refer to cases where findings differed by more than 10% between pseudocolor images and FAF images.
Normal = no pathologies in the posterior chamber were noted during clinical examination.
A disagreement for the grading of FAF images versus pseudocolor images in at least one quadrant was seen in 90 eyes (19.5%), of which 49 eyes demonstrated discrepancies in two or more quadrants (Fig. 2). There was no significant difference between the disease groups regarding agreement between the two modalities.
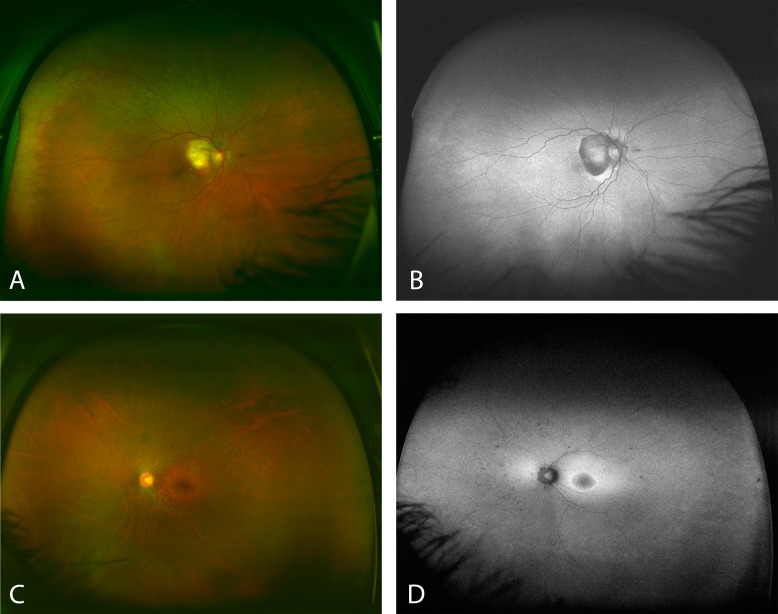
Figure 2. .
Images (A) and (B) show a case with AMD that appears to have almost no peripheral abnormalities on FAF (B), but the abnormalities seem more evident in the pseudocolor image (A). Contrarily, (C) and (D) show a case with a retinal dystrophy. The pseudocolor image (C) does not suggest noticeable peripheral abnormalities. However, they become more clear on the FAF image (D).
Intergrader Agreement
Agreement between the two graders was excellent, with kappa scores of 0.97 and 0.92 for FAF and pseudocolor images, respectively. However, there were 17 cases that differed in grading by more than two sectors. Moreover, three FAF images and seven pseudocolor images were graded differently in all four quadrants. One case was deemed ungradable by one grader but not so by the other grader. Agreement was slightly worse when only considering cases with at least one but fewer than four quadrants involved with kappa values of 0.95 for FAF images and 0.85 for pseudocolor images.
Discussion
In this study we present the prevalence and initial characterization of peripheral fundus autofluorescence changes in a retinal clinic population at a tertiary care academic center. Not surprisingly, we found a very high prevalence of peripheral changes in our patient cohort, regardless of whether FAF or pseudocolor images were analyzed. Overall, there was a strong correlation between abnormalities graded on FAF and pseudocolor images, suggesting that most visible fundus changes also appear on FAF images. Correlates have already been described for posterior pole findings, linking features such as retinal pigment epithelium (RPE) hypertrophy, RPE atrophy, hemorrhage, and drusen visible on color photographs and biomicroscopy with areas of abnormal fluorescence on FAF images.14 However, the visibility of these features may vary significantly between the color and FAF imaging. As such, there was a significant number of cases with grading discrepancies between pseudocolor and FAF, with 49 eyes showing differences in two or more quadrants. Careful rereview of these cases revealed that the peripheral abnormalities were very subtle (Fig. 2). Overall, however, excellent intergrader reproducibility confirmed that most peripheral abnormalities could be detected reliably and consistently.
By direct comparison, grading of FAF images seemed to yield better reproducibility results than pseudocolor grading. In part this may be due to the high-contrast nature of FAF images, potentially establishing an easier and more uniform threshold for identifying abnormalities. The peripheral abnormalities on the FAF images can, by definition, only manifest as areas of hyper- or hypofluorescence, whereas the spectrum of potential abnormality on pseudocolor images may be considerably larger. In addition, although a good correlation between abnormalities on standard color photographs and red–green pseudocolor SLO images has been previously reported,15 the reduced color information (including absence of the blue channel) in pseudocolor images may also adversely affect the reproducibility of abnormality detection with this modality.
One of the most interesting findings from our study is the high prevalence of peripheral abnormalities in patients with diseases generally thought to be macular diseases. For example, this prevalence exceeded 70% in eyes with AMD and 50% in eyes with CSR, both on pseudocolor and FAF images. By comparison, eyes without any clinically diagnosed retinal disease showed peripheral abnormalities in only approximately 18% of eyes. The age of the patient may have been a significant contributor to the high prevalence of peripheral abnormalities, because patients with AMD were significantly older than subjects without ophthalmoscopically apparent retinal disease. However, the nature and relevance of peripheral changes in AMD have received noticeable attention recently.16,17 The eyes mentioned above without retinal disease but peripheral abnormalities were most likely found to have slight peripheral changes on funduscopy not deemed worthy of being given a clinical diagnosis. It is also possible that media opacities such as cataracts may have given the false impression of a peripheral abnormality on the SLO images. In any case, attention should be given to peripheral changes even in these cases, although only long-term longitudinal studies may shed some light into their significance.
Analysis of the location of the peripheral findings did not reveal a specific pattern or clear trend, in that the changes seemed to be distributed relatively uniformly and did not show disease-specific predilection for specific sectors. A large number of eyes (35.3%) demonstrated peripheral changes in all four quadrants. The assessment of the distribution and location of peripheral FAF abnormalities, however, was confounded to some extent by the variable visualization of specific sectors. For example, more peripheral portions of the nasal and temporal fundus were often seen compared with the superior and inferior retina, due to interference in the visualization of the inferior and superior retina by the lids.
The number of eyes found to be ungradable in this series was quite low (1.9%), with a slight bias toward eyes with vascular or inflammatory retinal diseases. The most common cause of insufficient image quality was severe media opacity. Interestingly, FAF images seemed to be more affected by media opacity, because only four eyes had ungradable pseudocolor images and in all these four eyes the FAF images were also deemed to be ungradable. Overall, however, image quality did not seem to be a significant confounder in the analysis of ultra-widefield images.
There are a number of limitations to our study. First, the analysis was retrospective and, thus, imaging procedures and other clinical data were not necessarily acquired in a standardized fashion (e.g., not every patient has both pseudocolor and FAF images). As a result, for correlative analyses, many eyes had to be excluded to homogenize the data set, thus introducing a potential selection bias. In addition, the patients imaged in this study were drawn from a tertiary care retinal practice, and could potentially have had more severe disease and a higher prevalence of abnormalities compared with the general population. Thus, the prevalence data in this cohort may constitute an overestimate and may not generalize to other populations. Moreover, although findings in pseudocolor and FAF images were compared, there was no direct comparison of pseudocolor images with biomicroscopy or standard white-flash color photographs. Of note, standard color photography could not be used for comparison in this study, largely because the far peripheral retina could not be consistently imaged with this approach, precluding a direct comparison of findings. It is unclear how the artificial color rendering in pseudocolor images may have affected the grading findings.
Despite these limitations, our study also has several strengths. First, the grading was done according to a standardized protocol and the reproducibility of the assessments was confirmed by comparison of masked, independent assessments of two graders. Second, a relatively wide spectrum of retinal diseases was included in the analysis. Finally, the series was relatively large, including over 300 eyes with both FAF and pseudocolor images.
In summary, we observed that peripheral FAF abnormalities were common and present in the majority of eyes in this cohort of patients undergoing imaging in a tertiary care retinal practice. Peripheral abnormalities were often widespread, involving all quadrants of the retina, and frequent even in eyes with diseases thought to be primarily macular disorders. Although peripheral FAF abnormalities correlated well with RPE alterations visible on pseudocolor images, there were notable differences in some cases, suggesting that the modalities provided complementary information. Importantly, peripheral abnormalities on both pseudocolor and FAF images could be graded reproducibly, suggesting that these features are suitable for objective and standardized evaluation. The clinical relevance or significance of these abnormalities, however, remains to be shown in future prospective, longitudinal studies.
Footnotes
Supported in part by National Eye Institute/National Institutes of Health Grants EY03040 and R01 EY014375 and Deutsche Forschungsgemeinschaft Grant He 6094/1-1.
Disclosure: F.M. Heussen, None; C.S. Tan, None; S.R. Sadda, Carl Zeiss Meditec (F), Optovue (F), Optos (F), Heidelberg Engineering (C), P
References
- 1.Hyams SW, Neumann E, Friedman Z. Myopia-aphakia. II. Vitreous and peripheral retina. Br J Ophthalmol. 1975;59:483–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai TYY, Fan DSP, Lai WWK, Lam DSC. Peripheral and posterior pole retinal lesions in association with high myopia: a cross-sectional community-based study in Hong Kong. Eye. 2008;22:209–213 [DOI] [PubMed] [Google Scholar]
- 3.Coffee RE, Jain A, McCannel TA. Ultra wide-field imaging of choroidal metastasis secondary to primary breast cancer. Semin Ophthalmol. 2009;24:34–36 [DOI] [PubMed] [Google Scholar]
- 4.Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical, angiographic, and histologic study. AJOPHT. 2009;148:932–938 [DOI] [PubMed] [Google Scholar]
- 5.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866 [PubMed] [Google Scholar]
- 6.Charbel IP, Berendschot TTJM, Staurenghi G, Holz FG, Scholl HPN. Confocal blue reflectance imaging in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2008;49:1172–1177 [DOI] [PubMed] [Google Scholar]
- 7.Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538 [DOI] [PubMed] [Google Scholar]
- 8.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HPN, Schmitz-Valckenberg S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472 [DOI] [PubMed] [Google Scholar]
- 9.von Rückmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995;79:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003;110:392–399 [DOI] [PubMed] [Google Scholar]
- 11.Schmitz-Valckenberg S, Fleckenstein M, Göbel AP, et al. Evaluation of autofluorescence imaging with the scanning laser ophthalmoscope and the fundus camera in age-related geographic atrophy. Am J Ophthalmol. 2008;146:183–192 [DOI] [PubMed] [Google Scholar]
- 12.Neubauer AS, Kernt M, Haritoglou C, Priglinger SG, Kampik A, Ulbig MW. Nonmydriatic screening for diabetic retinopathy by ultra-widefield scanning laser ophthalmoscopy (Optomap). Graefes Arch Clin Exp Ophthalmol. 2008;246:229–235 [DOI] [PubMed] [Google Scholar]
- 13.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D'Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409 [DOI] [PubMed] [Google Scholar]
- 15.Heussen FM, Vasconcelos-Santos DV, Pappuru RR, Walsh AC, Rao NA, Sadda SR. Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging. 2011;42:272–277 [DOI] [PubMed] [Google Scholar]
- 16.Shuler RK, Schmidt S, Gallins P, et al. Peripheral reticular pigmentary change is associated with complement factor H polymorphism (Y402H) in age-related macular degeneration. Ophthalmology. 2008;86:520–524 [DOI] [PubMed] [Google Scholar]
- 17.Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2011;50:586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]