Abstract
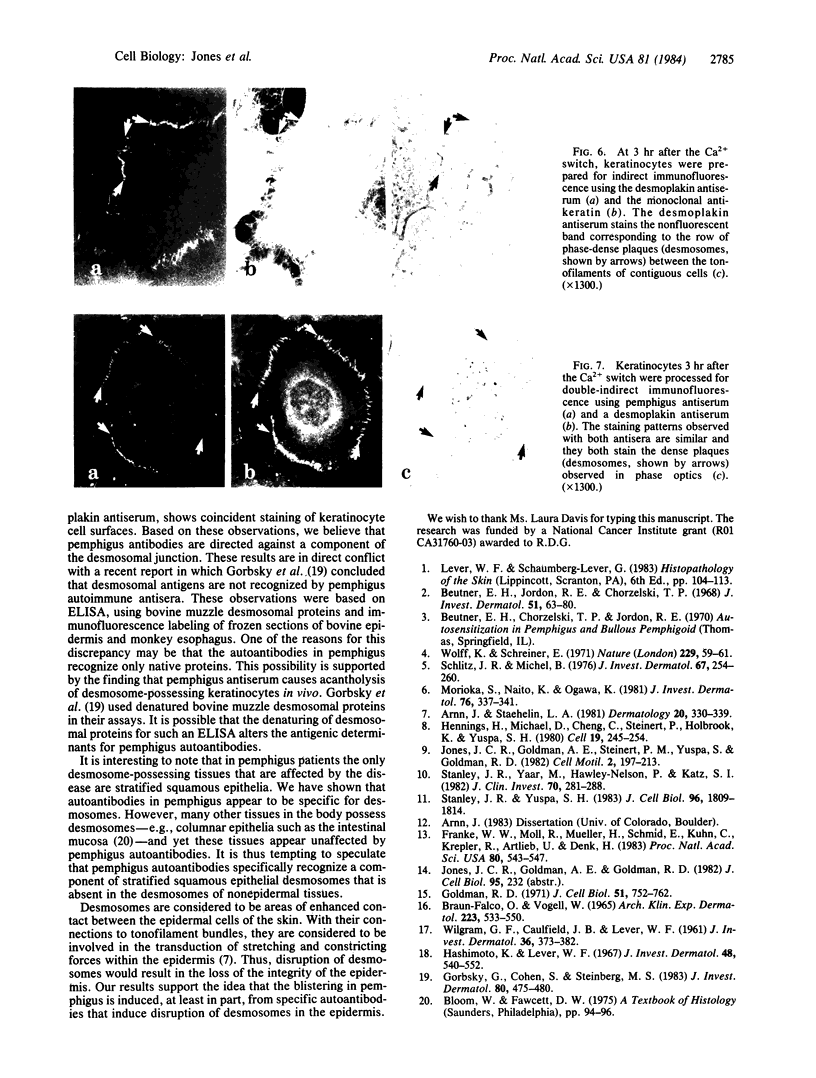
Pemphigus is a human disease that causes extensive blistering of the skin. This blistering is related to a loss of epidermal cell cohesion and is accompanied by circulating autoantibodies that stain epidermal cell surfaces, as shown by immunofluorescence microscopy. One of the major components involved in epidermal cell cohesion is the desmosome. The pathological changes that accompany pemphigus led us to determine whether the autoantibodies are specific for desmosomes. Incubation of cultured mouse keratinocytes in medium containing pemphigus antiserum leads to cell separation at cell-cell contact sites, which possess desmosomes. Tissue sections of mouse skin processed for indirect immunofluorescence, using pemphigus antiserum or a rabbit antiserum directed against components of desmosomes, show similar punctate cell-surface staining patterns within the epidermis. Cultured mouse keratinocytes possessing well-defined intermediate filament bundles (tonofilaments) and desmosomes were processed for double indirect immunofluorescence, using a monoclonal antibody directed against mouse skin keratin and either pemphigus antiserum or the desmosome antiserum. The keratinocytes exhibit a complex system of keratin-containing tonofilaments. Tonofilaments in contacting cells are separated by thin dark bands at the cell surface, which correspond precisely to desmosomal plaques seen by phase-contrast microscopy. These bands specifically stain with both pemphigus antiserum and the desmosome antiserum. Double indirect immunofluorescence of the cultured mouse keratinocytes, using pemphigus antiserum and the desmosome antiserum, reveals that the pemphigus autoantibodies stain the same areas of cell-cell contact as the desmosome antibodies. Our evidence supports the idea that pemphigus blisters form, at least in part, from a specific antibody-induced disruption of desmosomes in the epidermis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnn J., Staehelin L. A. The structure and function of spot desmosomes. Int J Dermatol. 1981 Jun;20(5):330–339. doi: 10.1111/j.1365-4362.1981.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Beutner E. H., Jordon R. E., Chorzelski T. P. The immunopathology of pemphigus and bullous pemphigoid. J Invest Dermatol. 1968 Aug;51(2):63–80. [PubMed] [Google Scholar]
- Braun-Falco O., Vogell W. Elektronenmikroskopische Untersuchungen zur Dynamik der Akantholyse bei Pemphigus vulgaris. II. Die akantholytische Blase. Arch Klin Exp Dermatol. 1965 Dec 16;223(6):533–550. [PubMed] [Google Scholar]
- Franke W. W., Moll R., Mueller H., Schmid E., Kuhn C., Krepler R., Artlieb U., Denk H. Immunocytochemical identification of epithelium-derived human tumors with antibodies to desmosomal plaque proteins. Proc Natl Acad Sci U S A. 1983 Jan;80(2):543–547. doi: 10.1073/pnas.80.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G., Cohen S., Steinberg M. S. Desmosomal antigens are not recognized by the majority of pemphigus autoimmune sera. J Invest Dermatol. 1983 Jun;80(6):475–480. doi: 10.1111/1523-1747.ep12534924. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Lever W. F. An electron microscopic study on pemphigus vulgaris of the mouth and the skin with special reference to the intercellular cement. J Invest Dermatol. 1967 Jun;48(6):540–552. doi: 10.1038/jid.1967.86. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Steinert P. M., Yuspa S., Goldman R. D. Dynamic aspects of the supramolecular organization of intermediate filament networks in cultured epidermal cells. Cell Motil. 1982;2(3):197–213. doi: 10.1002/cm.970020302. [DOI] [PubMed] [Google Scholar]
- Morioka S., Naito K., Ogawa H. The pathogenic role of pemphigus antibodies and proteinase in epidermal acantholysis. J Invest Dermatol. 1981 May;76(5):337–341. doi: 10.1111/1523-1747.ep12519988. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976 Aug;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Yaar M., Hawley-Nelson P., Katz S. I. Pemphigus antibodies identify a cell surface glycoprotein synthesized by human and mouse keratinocytes. J Clin Invest. 1982 Aug;70(2):281–288. doi: 10.1172/JCI110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. R., Yuspa S. H. Specific epidermal protein markers are modulated during calcium-induced terminal differentiation. J Cell Biol. 1983 Jun;96(6):1809–1814. doi: 10.1083/jcb.96.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILGRAM G. F., CAULFIELD J. B., LEVER W. F. An electron microscopic study of acantholysis in pemphigus vulgaris. J Invest Dermatol. 1961 May;36:373–382. doi: 10.1038/jid.1961.58. [DOI] [PubMed] [Google Scholar]
- Wolff K., Schreiner E. Ultrastructural localization of pemphigus autoantibodies within e epidermis. Nature. 1971 Jan 1;229(5279):59–61. doi: 10.1038/229059a0. [DOI] [PubMed] [Google Scholar]









