Abstract
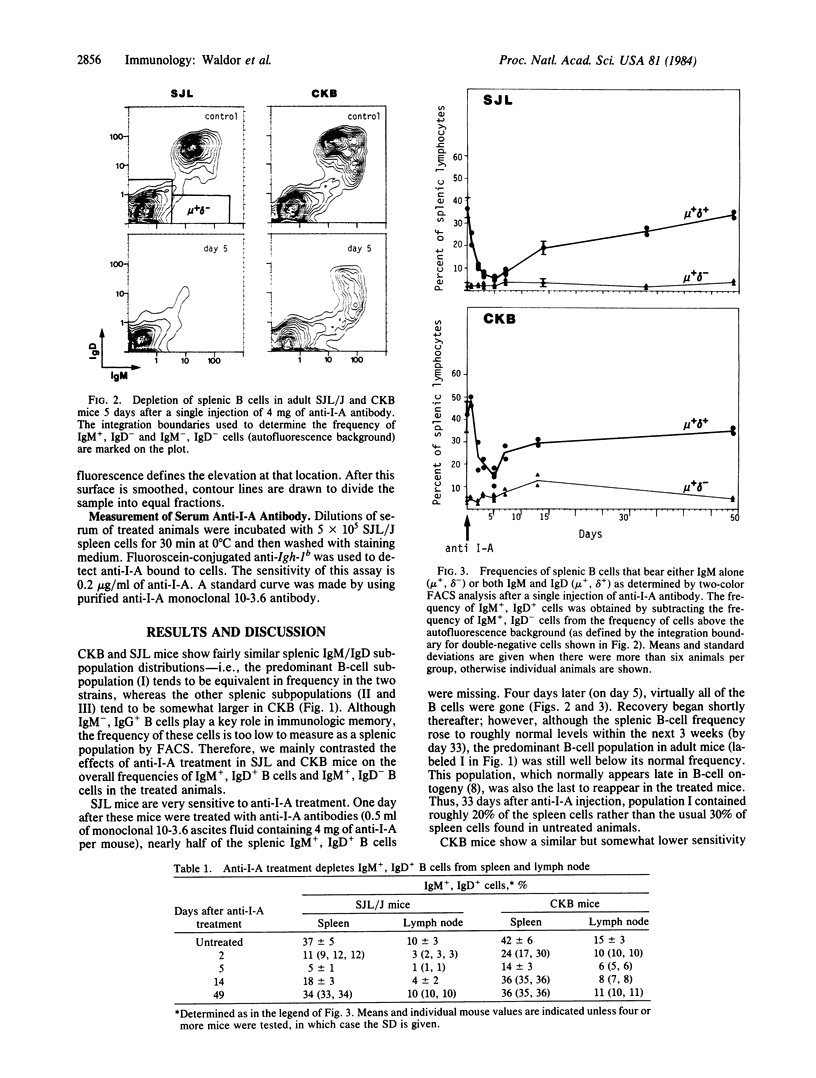
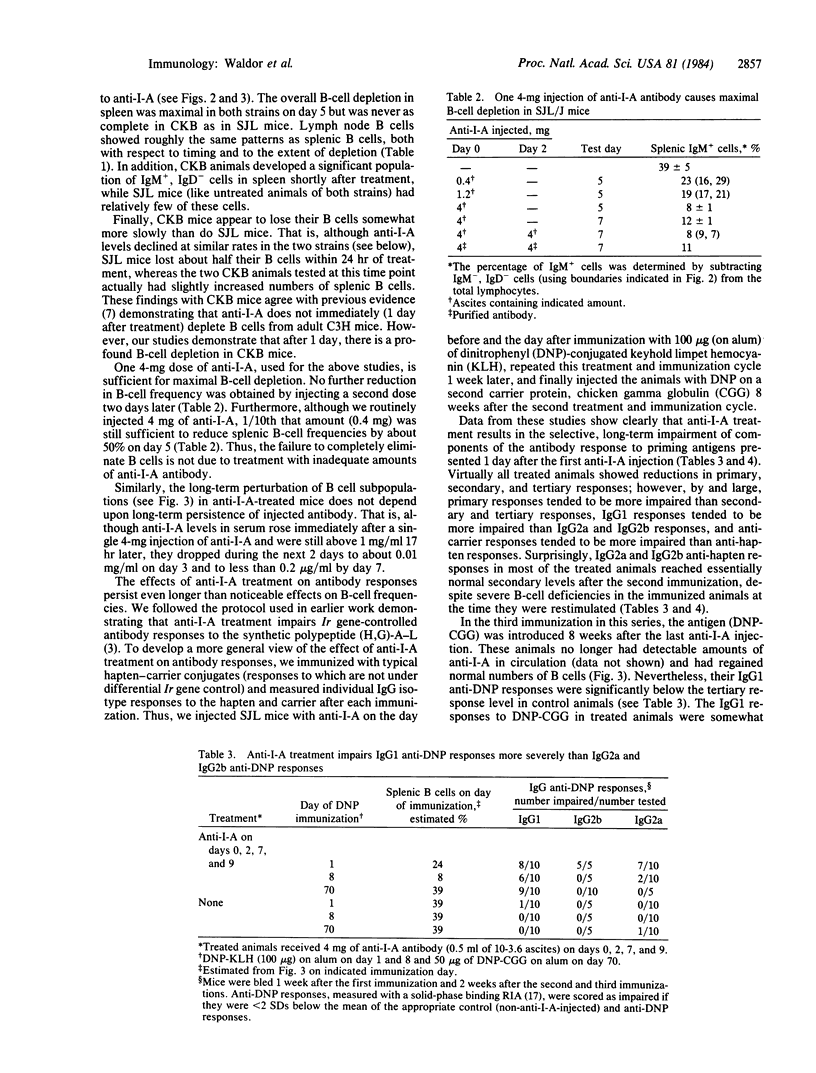
Previous studies have shown that treatment with antibodies to the murine I-A antigen encoded in the major histocompatibility complex attenuates experimental allergic encephalitis and experimental autoimmune myasthenia gravis. These studies were conducted with SJL mice, an inbred strain that is highly susceptible to the induction of these diseases. Here we show that injection of monoclonal anti-I-A antibody in the amounts used for the above studies rapidly depletes B cells. Fluorescence-activated cell sorter (FACS) multiparameter analysis of the B-cell subpopulations in treated animals shows that maximum depletion occurs around 5 days after treatment and that recovery of some subpopulations i still incomplete 1 month later. SJL mice are more sensitive to this B-cell depletion and recover more slowly than putatively normal C3H.Ighb (CKB) mice. Some components of the primary, secondary and tertiary IgG antibody responses are reduced in anti-I-A-treated SJL animals immunized after the first and second anti-I-A injections. The persistence of some antibody response impairment well beyond the time when anti-I-A disappears raises a note of caution concerning human therapy protocols based on the injection of anti-Ia antibodies.
Full text
PDF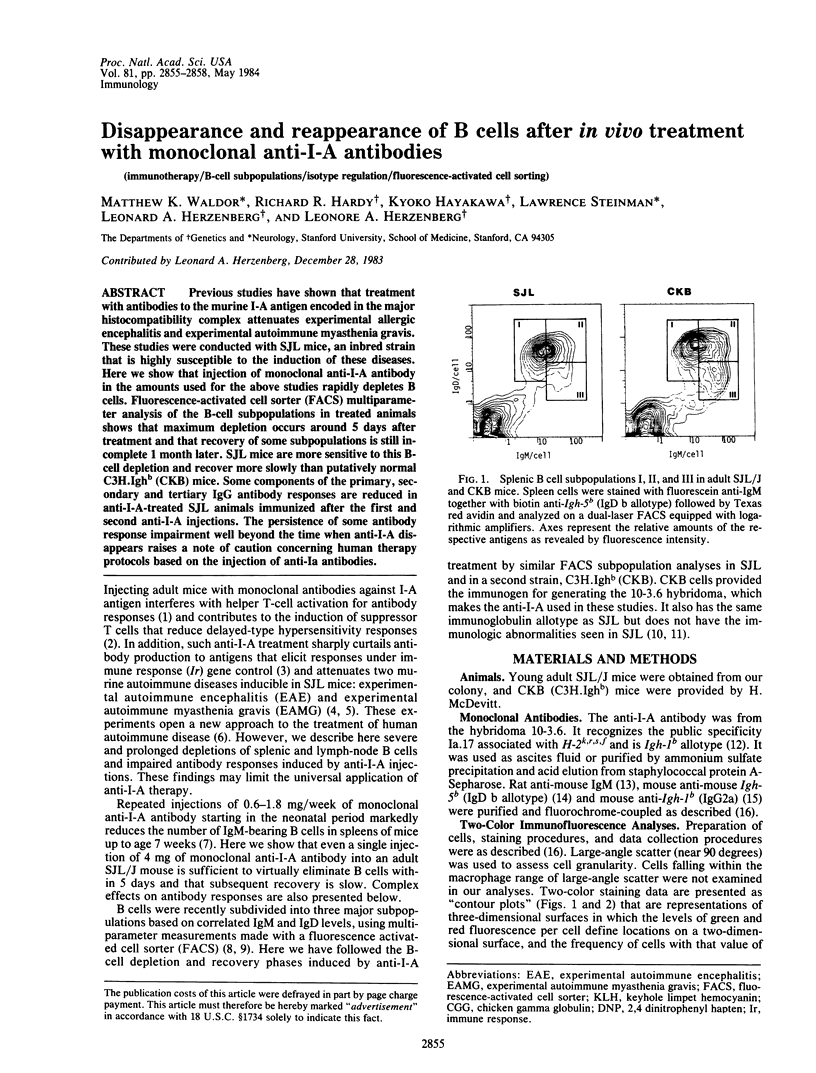

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fultz M. J., Scher I., Finkelman F. D., Kincade P., Mond J. J. Neonatal suppression with anti-Ia antibody. I. Suppression of murine B lymphocyte development. J Immunol. 1982 Sep;129(3):992–995. [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Haaijman J., Herzenberg L. A. B-cell subpopulations identifiable by two-color fluorescence analysis using a dual-laser FACS. Ann N Y Acad Sci. 1982;399:112–121. doi: 10.1111/j.1749-6632.1982.tb25667.x. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Haaijman J., Herzenberg L. A. B-cell subpopulations identified by two-colour fluorescence analysis. Nature. 1982 Jun 17;297(5867):589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Short-term and chronic allotype suppression in mice. Contemp Top Immunobiol. 1974;3:41–75. doi: 10.1007/978-1-4684-3045-5_2. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Tokuhisa T., Hayakawa K. Epitope-specific regulation. Annu Rev Immunol. 1983;1:609–632. doi: 10.1146/annurev.iy.01.040183.003141. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Suppressing autoimmunity in mice. Science. 1983 Aug 26;221(4613):843–845. doi: 10.1126/science.6576470. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Greene M. I. Conversion of immunity to suppression by in vivo administration of I-A subregion-specific antibodies. J Exp Med. 1982 Aug 1;156(2):480–491. doi: 10.1084/jem.156.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Adelman N. E., McDevitt H. O. In vivo effects of antibodies to immune response gene products. I. Haplotype-specific suppression of humoral immune responses with a monoclonal anti-I-A. J Exp Med. 1981 Nov 1;154(5):1694–1702. doi: 10.1084/jem.154.5.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Effects of blocking helper T cell induction in vivo with anti-Ia antibodies. Possible role of I-A/E hybrid molecules as restriction elements. J Exp Med. 1980 Oct 1;152(4):996–1010. doi: 10.1084/jem.152.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Steinman L., Rosenbaum J. T., Sriram S., McDevitt H. O. In vivo effects of antibodies to immune response gene products: prevention of experimental allergic encephalitis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7111–7114. doi: 10.1073/pnas.78.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., McDevitt H. O., Steinman L. In vivo therapy with monoclonal anti-I-A antibody suppresses immune responses to acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 May;80(9):2713–2717. doi: 10.1073/pnas.80.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanebo H. J., Gallmeier W. M., Boyse E. A., Old L. J. Paraproteinemia and reticulum cell sarcoma in an inbred mouse strain. Science. 1966 Nov 18;154(3751):901–903. doi: 10.1126/science.154.3751.901. [DOI] [PubMed] [Google Scholar]



