Abstract
Analyses by differential centrifugation of liver homogenates from rats that had received 131I-labeled asialoorosomucoid showed that, 1 min after injection, most of the intracellular ligand was associated with a particle that did not sediment at 2.5 X 10(5) g-min. However, by 10 min, undigested ligand became associated with a particle that did sediment at this speed. On analytical ultracentrifugation in sucrose gradients, both kinds of particles exhibited low densities (1.11-1.13 g X ml-1). In contrast to asialoorosomucoid, 125I-labeled asialotransferrin type 3, under noncatabolic conditions, remained largely confined to the nonsedimenting particle regardless of the duration of the study. Induction of catabolism of asialotransferrin was accompanied by the appearance of the ligand in the sedimentable particle. The nonsedimentable particle was separated by immunoadsorption from other subcellular particles contained in the low-density subcellular fraction. The adsorbant , prepared by immobilizing purified antibodies to the Gal/GalN-specific lectin from rat liver on coated polyacrylamide beads, removed 75-80% of the asialoorosomucoid and transferrin binding capacities present, together with a similar portion of the radioligands tested (asialoorosomucoid, asialotransferrin type 3, and human diferric transferrin). Significantly, the sialytransferase activity remained unadsorbed. From these findings, the nonsedimentable particle appears to be involved in the transport of ligands destined to such diverse fates as exocytosis or lysosomal degradation. The sedimentable particle, on the other hand, seems to represent a link between the first particle and the lysosome.
Full text
PDF
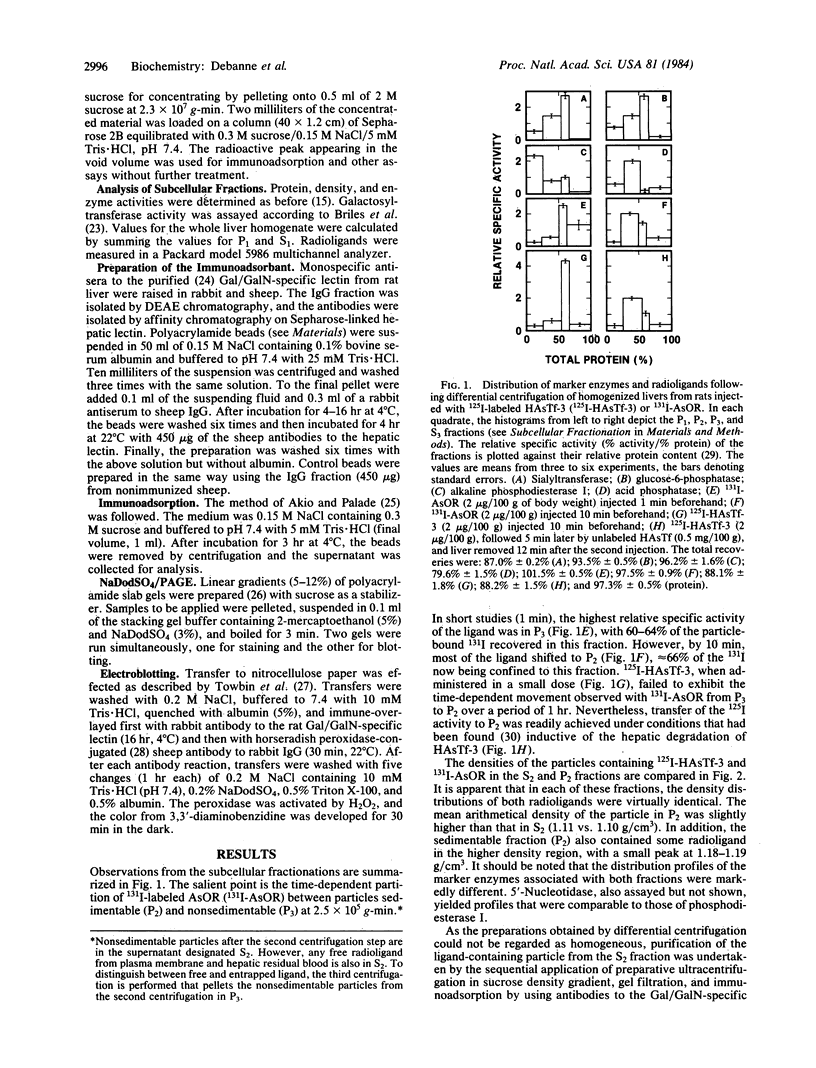
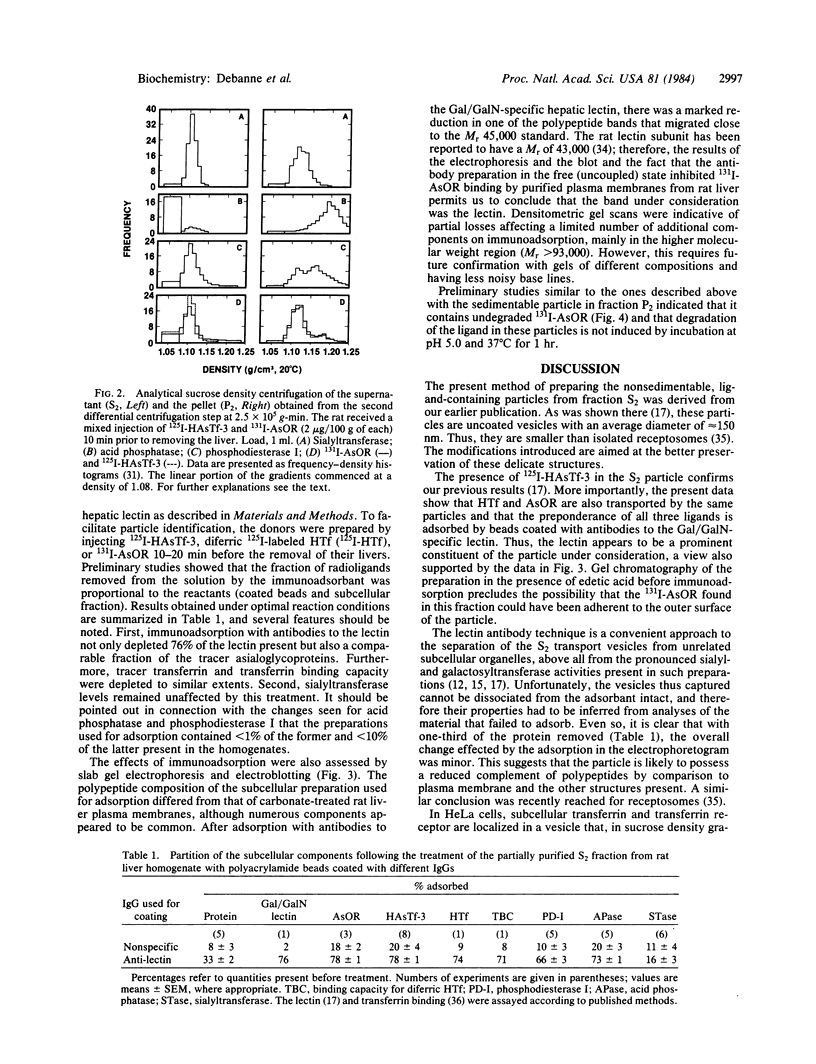
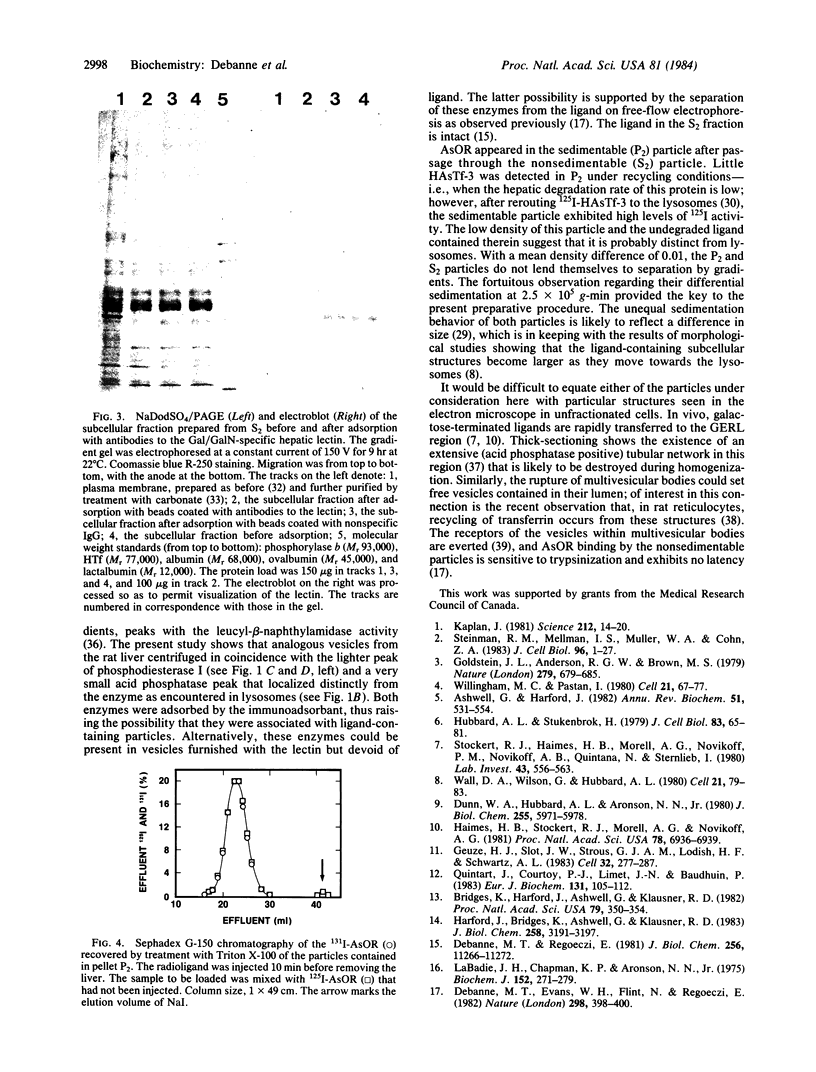

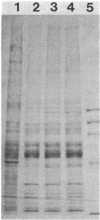
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges K., Harford J., Ashwell G., Klausner R. D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Li E., Kornfeld S. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J Biol Chem. 1977 Feb 10;252(3):1107–1116. [PubMed] [Google Scholar]
- Charlwood P. A., Regoeczi E., Hatton M. W. Hepatic uptake and degradation of trace doses of asialofetuin and asialoorosomucoid in the intact rat. Biochim Biophys Acta. 1979 Jun 1;585(1):61–70. doi: 10.1016/0304-4165(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Evans W. H., Flint N., Regoeczi E. Receptor-rich intracellular membrane vesicles transporting asialotransferrin and insulin in liver. Nature. 1982 Jul 22;298(5872):398–400. doi: 10.1038/298398a0. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Regoeczi E., Hatton M. W. Multivalent interaction between asialofetuin and plasma membrane preparations from the rat liver. Can J Biochem. 1980 Dec;58(12):1414–1420. doi: 10.1139/o80-191. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Regoeczi E. Subcellular distribution of human asialotransferrin type 3 in the rat liver. J Biol Chem. 1981 Nov 10;256(21):11266–11272. [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Duve C. Exploring cells with a centrifuge. Science. 1975 Jul 18;189(4198):186–194. doi: 10.1126/science.1138375. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Haimes H. B., Stockert R. J., Morell A. G., Novikoff A. B. Carbohydrate-specified endocytosis: localization of ligand in the lysosomal compartment. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6936–6939. doi: 10.1073/pnas.78.11.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J., Bridges K., Ashwell G., Klausner R. D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J Biol Chem. 1983 Mar 10;258(5):3191–3197. [PubMed] [Google Scholar]
- Harford J., Lowe M., Tsunoo H., Ashwell G. Immunological approaches to the study of membrane receptors. A monoclonal antibody that inhibits the binding of asialoglycoproteins to the rat liver receptor. J Biol Chem. 1982 Nov 10;257(21):12685–12690. [PubMed] [Google Scholar]
- Hubbard A. L., Ma A. Isolation of rat hepatocyte plasma membranes. II. Identification of membrane-associated cytoskeletal proteins. J Cell Biol. 1983 Jan;96(1):230–239. doi: 10.1083/jcb.96.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. II. Intracellular fates of the 125I-ligands. J Cell Biol. 1979 Oct;83(1):65–81. doi: 10.1083/jcb.83.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Ito A., Palade G. E. Presence of NADPH-cytochrome P-450 reductase in rat liver Golgi membranes. Evidence obtained by immunoadsorption method. J Cell Biol. 1978 Nov;79(2 Pt 1):590–597. doi: 10.1083/jcb.79.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Polypeptide-binding membrane receptors: analysis and classification. Science. 1981 Apr 3;212(4490):14–20. doi: 10.1126/science.6259730. [DOI] [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Limet J. N., Baudhuin P. Galactose-specific endocytosis in rat liver. Biochemical and morphological characterization of a low-density compartment isolated from hepatocytes. Eur J Biochem. 1983 Mar 1;131(1):105–112. doi: 10.1111/j.1432-1033.1983.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Chindemi P. A., Debanne M. T., Hatton M. W. Dual nature of the hepatic lectin pathway for human asialotransferrin type 3 in the rat. J Biol Chem. 1982 May 25;257(10):5431–5436. [PubMed] [Google Scholar]
- Regoeczi E. Iodogen-catalyzed iodination of transferrin. Int J Pept Protein Res. 1983 Oct;22(4):422–433. doi: 10.1111/j.1399-3011.1983.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Taylor P., Debanne M. T., März L., Hatton M. W. Three types of human asialo-transferrin and their interactions with the rat liver. Biochem J. 1979 Nov 15;184(2):399–407. doi: 10.1042/bj1840399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Haimes H. B., Morell A. G., Novikoff P. M., Novikoff A. B., Quintana N., Sternlieb I. Endocytosis of asialoglycoprotein-enzyme conjugates by hepatocytes. Lab Invest. 1980 Dec;43(6):556–563. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]