Abstract
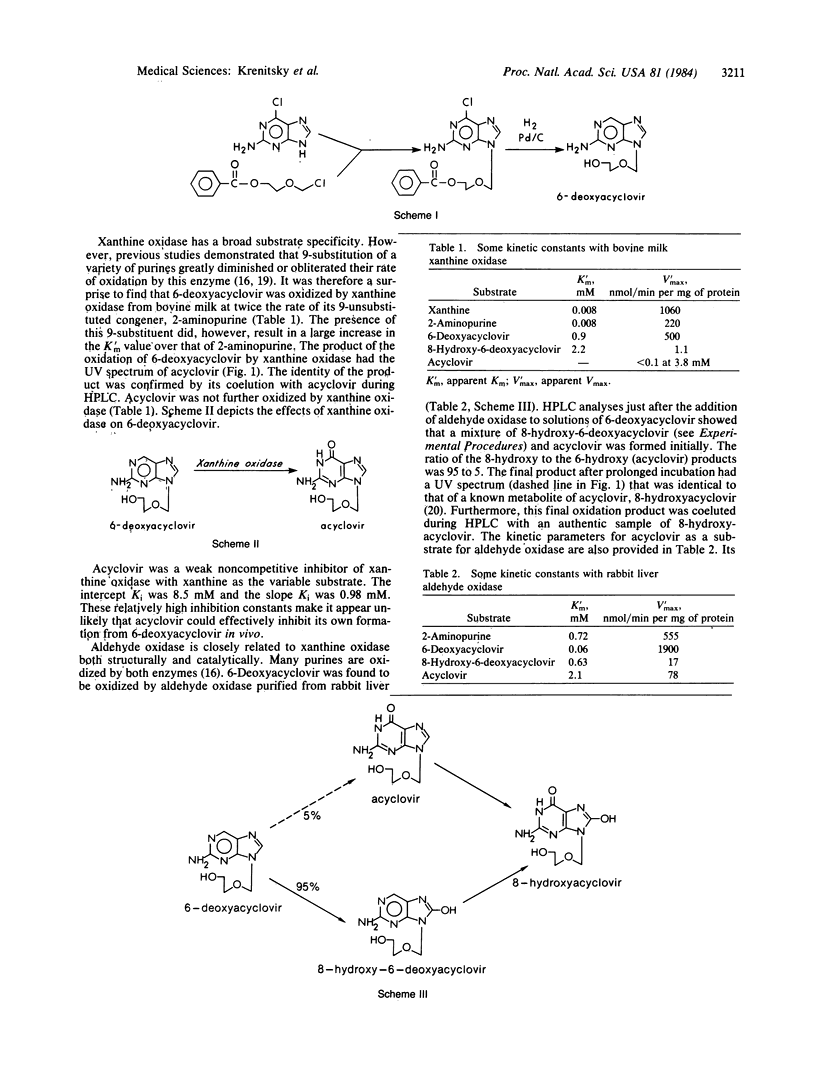
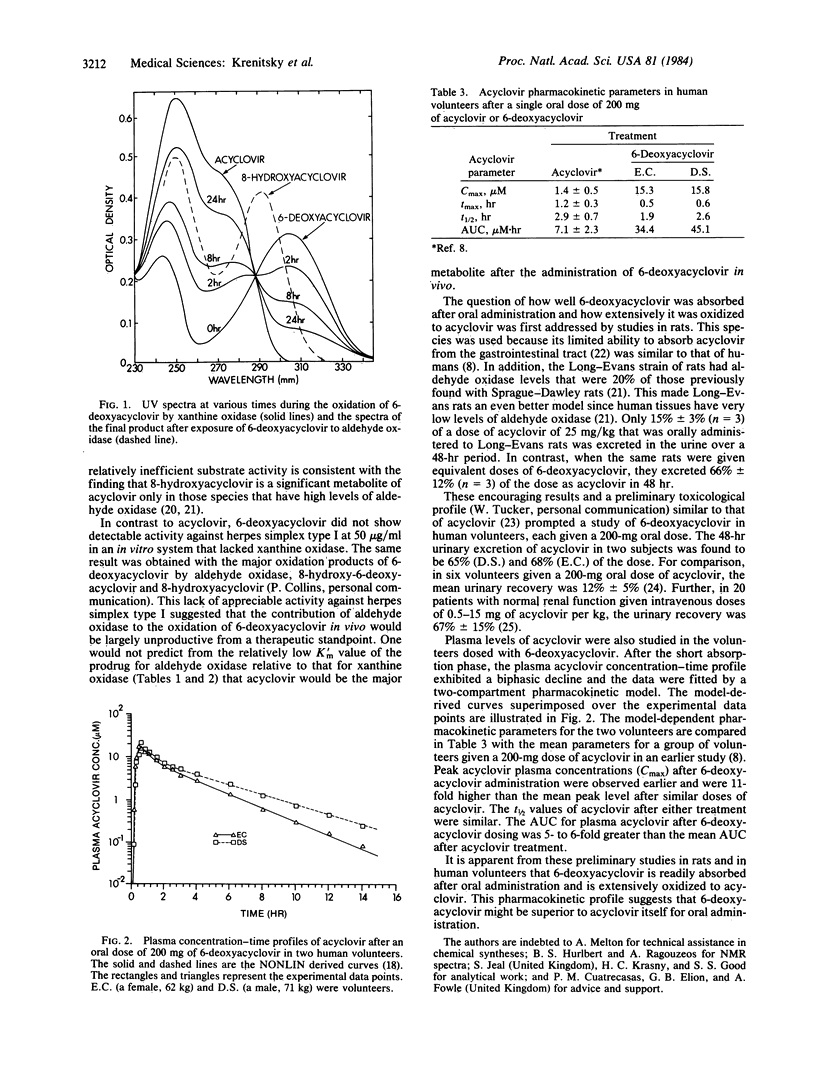
Acyclovir [9-[(2-hydroxyethoxy)methyl]guanine] is an acyclic guanine nucleoside analogue that is widely used clinically as an antiherpetic agent. Its limited absorption in humans after oral administration prompted the search for prodrugs. A congener, referred to as 6- deoxyacyclovir [2-amino-9-[(2-hydroxyethoxy)methyl]-9H-purine], was synthesized and found to be 18 times more water soluble than was acyclovir. Surprisingly, this congener was readily oxidized to acyclovir by xanthine oxidase (EC 1.2.3.2). It was also oxidized by aldehyde oxidase (EC 1.2.3.1) largely to 8-hydroxy-6- deoxyacyclovir [2-amino-8-hydroxy-9-[(2-hydroxyethoxy)methyl]-9H-purine] and then to 8- hydroxyacyclovir [2-amino-6,8-dihydroxy-9[(2-hydroxyethoxy)methyl]-9H-purine]. 6- Deoxyacyclovir and the major products of its oxidation by aldehyde oxidase lacked appreciable activity against herpes simplex type I in vitro. On the basis of these results, it was apparent that the success of 6- deoxyacyclovir as a prodrug in vivo would depend upon how well its desired activation by xanthine oxidase competed with the nonactivating oxidations by aldehyde oxidase. In rats dosed orally with 6- deoxyacyclovir , absorption was extensive and the major urinary metabolite was acyclovir. In two human volunteers, urinary excretions of acyclovir were 5-6 times greater than those typically observed after administration of equivalent doses of acyclovir itself. The areas under the plasma concentration-time curves for acyclovir were also 5-6 times greater. Plasma levels of acyclovir peaked soon after ingestion of the prodrug, indicating rapid absorption and metabolic conversion. These results suggested that 6- deoxyacyclovir might have clinical usefulness as a prodrug of acyclovir suitable for oral administration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M. R., Liao S. H., de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. 1982 Jul 20;73(1A):186–192. doi: 10.1016/0002-9343(82)90088-2. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Colla L., De Clercq E., Busson R., Vanderhaeghe H. Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine]. J Med Chem. 1983 Apr;26(4):602–604. doi: 10.1021/jm00358a029. [DOI] [PubMed] [Google Scholar]
- Corey L., Benedetti J. K., Critchlow C. W., Remington M. R., Winter C. A., Fahnlander A. L., Smith K., Salter D. L., Keeney R. E., Davis L. G. Double-blind controlled trial of topical acyclovir in genital herpes simplex virus infections. Am J Med. 1982 Jul 20;73(1A):326–334. doi: 10.1016/0002-9343(82)90117-6. [DOI] [PubMed] [Google Scholar]
- Corey L., Fife K. H., Benedetti J. K., Winter C. A., Fahnlander A., Connor J. D., Hintz M. A., Holmes K. K. Intravenous acyclovir for the treatment of primary genital herpes. Ann Intern Med. 1983 Jun;98(6):914–921. doi: 10.7326/0003-4819-98-6-914. [DOI] [PubMed] [Google Scholar]
- Corey L., Nahmias A. J., Guinan M. E., Benedetti J. K., Critchlow C. W., Holmes K. K. A trial of topical acyclovir in genital herpes simplex virus infections. N Engl J Med. 1982 Jun 3;306(22):1313–1319. doi: 10.1056/NEJM198206033062201. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good S. S., Krasny H. C., Elion G. B., de Miranda P. Disposition in the dog and the rat of 2, 6-diamino-9-(2-hydroxyethoxymethyl)purine (A134U), a potential prodrug of acyclovir. J Pharmacol Exp Ther. 1983 Dec;227(3):644–651. [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1972 Jun;150(2):585–599. doi: 10.1016/0003-9861(72)90078-1. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Lettré H., Kapoor N. K., Werner D. Catabolism of some cytotoxic purine derivatives by xanthine oxidase and peroxidase. Biochem Pharmacol. 1967 Sep 9;16(9):1747–1755. doi: 10.1016/0006-2952(67)90250-x. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Wade J. C., Mitchell C. D., Saral R., Lietman P. S., Durack D. T., Levin M. J., Segreti A. C., Balfour H. H., Jr Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982 Jul 20;73(1A):229–235. doi: 10.1016/0002-9343(82)90097-3. [DOI] [PubMed] [Google Scholar]
- Quinn R. P., de Miranda P., Gerald L., Good S. S. A sensitive radioimmunoassay for the antiviral agent BW248U [9-(2-hydroxyethoxymethyl)guanine]. Anal Biochem. 1979 Oct 1;98(2):319–328. doi: 10.1016/0003-2697(79)90148-9. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., FRIDOVICH I., HANDLER P. Hepatic aldehyde oxidase. I. Purification and properties. J Biol Chem. 1962 Mar;237:922–928. [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Spector T., Jones T. E., Beacham L. M., 3rd Conversion of 2,6-diamino-9-(2-hydroxyethoxymethyl)purine to acyclovir as catalyzed by adenosine deaminase. Biochem Pharmacol. 1983 Sep 1;32(17):2505–2509. doi: 10.1016/0006-2952(83)90010-2. [DOI] [PubMed] [Google Scholar]
- Tucker W. E., Jr Preclinical toxicology profile of acyclovir: an overview. Am J Med. 1982 Jul 20;73(1A):27–30. doi: 10.1016/0002-9343(82)90058-4. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Blum M. R. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Krasny H. C., Page D. A., Elion G. B. The disposition of acyclovir in different species. J Pharmacol Exp Ther. 1981 Nov;219(2):309–315. [PubMed] [Google Scholar]


