Abstract
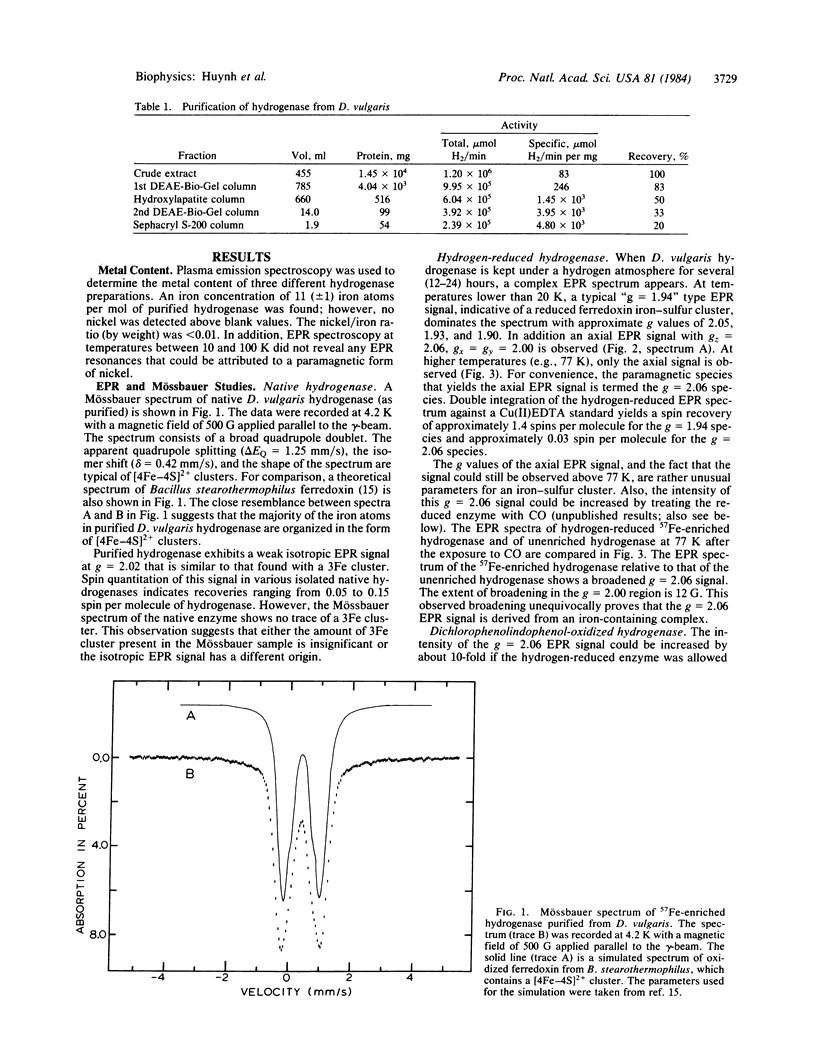
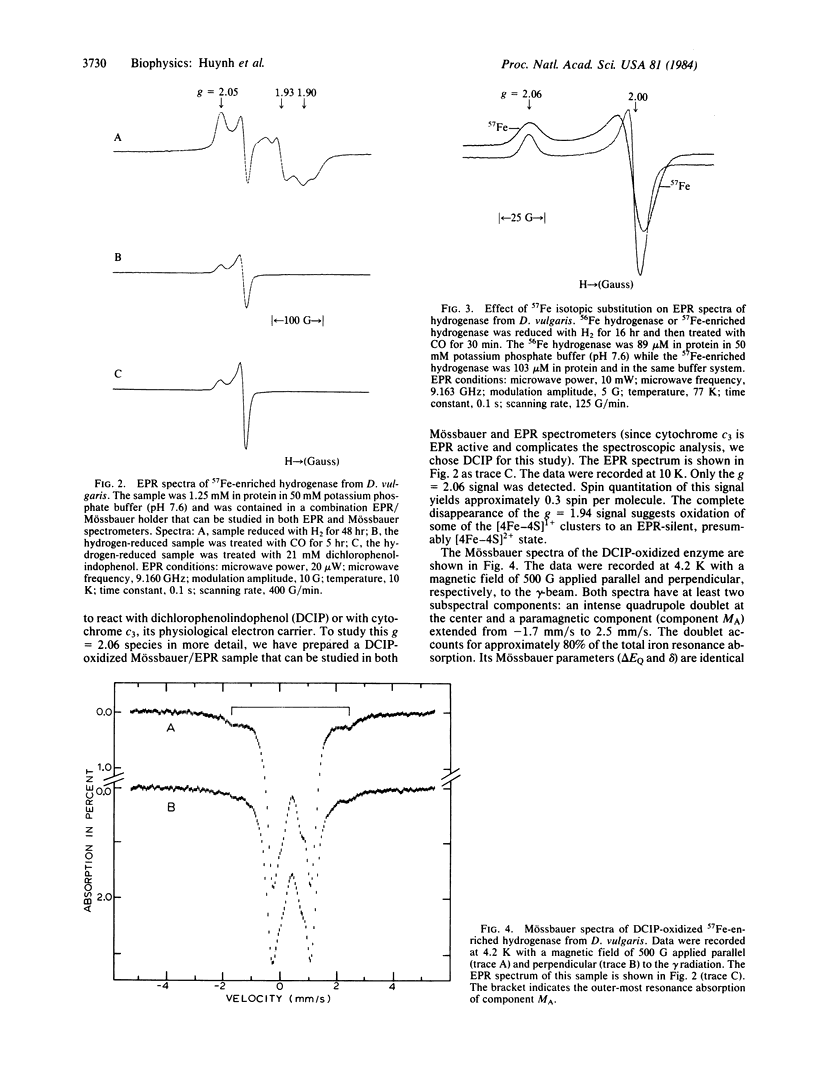
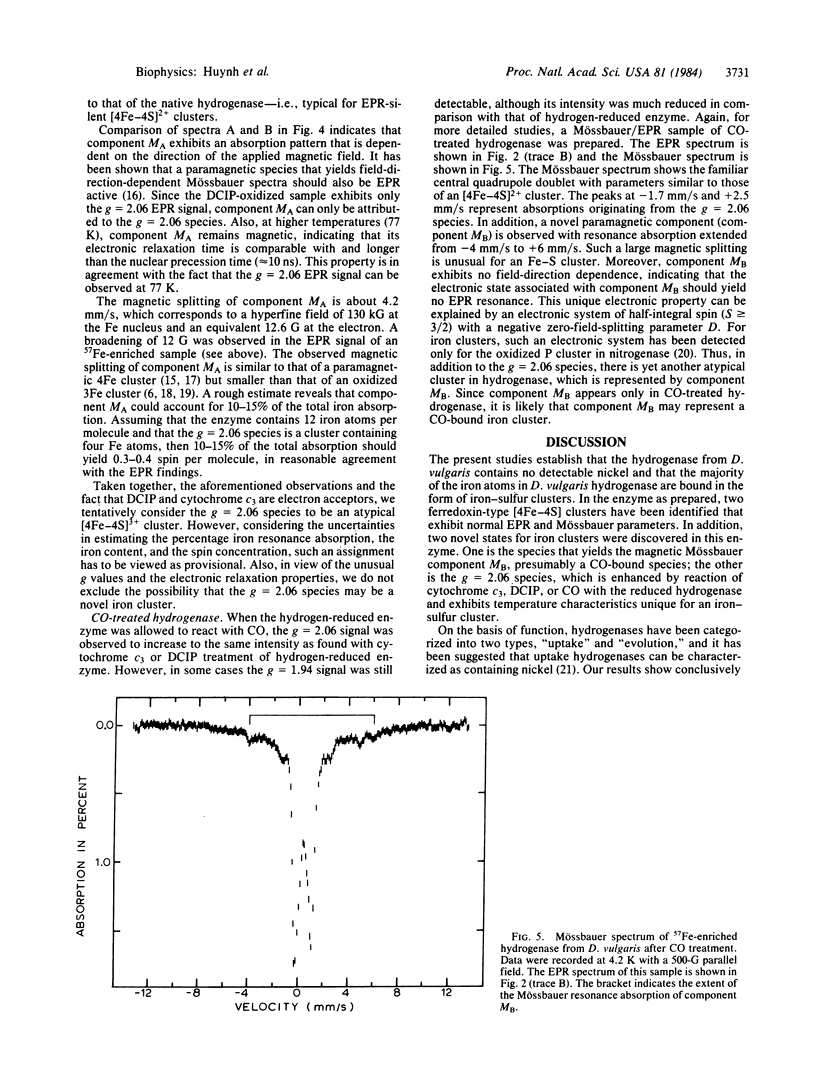
A purification procedure for the periplasmic hydrogenase from Desulfovibrio vulgaris ( Hildenborough , National Collection of Industrial Bacteria 8303) is reported. The purified hydrogenase has a specific activity of 4800 units per mg of protein. Plasma emission studies reveal that this highly active hydrogenase is free of nickel and contains 11 (+/- 1) nonheme iron atoms per molecule. A combined EPR and Mössbauer study indicates that the majority of the iron atoms are bound in the form of iron- sulfur clusters. Two ferredoxin-type [4Fe-4S] clusters have been identified that exhibit normal EPR and Mössbauer parameters; however, no trace of 3Fe cluster is detected by the Mössbauer measurement. In the presence of oxidants, cytochrome c3, and CO, anomalous EPR and Mössbauer spectra indicative of atypical nonheme iron centers are observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Emptage M. H., Kent T. A., Huynh B. H., Rawlings J., Orme-Johnson W. H., Münck E. On the nature of the iron-sulfur centers in a ferredoxin from Azotobacter vinelandii. Mössbauer studies and cluster displacement experiments. J Biol Chem. 1980 Mar 10;255(5):1793–1796. [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H., Orme-Johnson W. H. On the iron-sulfur cluster in hydrogenase from Clostridium pasteurianum W5. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4795–4799. doi: 10.1073/pnas.72.12.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Grande H. J., Dunham W. R., Averill B., Van Dijk C., Sands R. H. Electron paramagnetic resonance and other properties of hydrogenases isolated from Desulfovibrio vulgaris (strain Hildenborough) and Megasphaera elsdenii. Eur J Biochem. 1983 Oct 17;136(1):201–207. doi: 10.1111/j.1432-1033.1983.tb07727.x. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M., Le Gall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978 May 30;82(2):451–461. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Henzl M. T., Christner J. A., Zimmermann R., Orme-Johnson W. H., Münck E. Nitrogenase XII. Mössbauer studies of the MoFe protein from Clostridium pasteurianum W5. Biochim Biophys Acta. 1980 May 29;623(1):124–138. doi: 10.1016/0005-2795(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Lui M. C., Moura J. J., Moura I., Ljungdahl P. O., Münck E., Payne W. J., Peck H. D., Jr, DerVartanian D. V., LeGall J. Mössbauer and EPR studies on nitrite reductase from Thiobacillus denitrificans. J Biol Chem. 1982 Aug 25;257(16):9576–9581. [PubMed] [Google Scholar]
- Huynh B. H., Moura J. J., Moura I., Kent T. A., LeGall J., Xavier A. V., Münck E. Evidence for a three-iron center in a ferredoxin from Desulfovibrio gigas. Mössbauer and EPR studies. J Biol Chem. 1980 Apr 25;255(8):3242–3244. [PubMed] [Google Scholar]
- Krüger H. J., Huynh B. H., Ljungdahl P. O., Xavier A. V., Der Vartanian D. V., Moura I., Peck H. D., Jr, Teixeira M., Moura J. J., LeGall J. Evidence for nickel and a three-iron center in the hydrogenase of Desulfovibrio desulfuricans. J Biol Chem. 1982 Dec 25;257(24):14620–14623. [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- Middleton P., Dickson D. P., Johnson C. E., Rush J. D. Interpretation of the Mössbauer spectra of the four-iron ferredoxin from Bacillus stearothermophilus. Eur J Biochem. 1978 Jul 17;88(1):135–141. doi: 10.1111/j.1432-1033.1978.tb12430.x. [DOI] [PubMed] [Google Scholar]
- Middleton P., Dickson D. P., Johnson C. E., Rush J. D. Interpretation of the Mössbauer spectra of the high-potential iron protein from Chromatium. Eur J Biochem. 1980 Feb;104(1):289–296. doi: 10.1111/j.1432-1033.1980.tb04427.x. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Böcher R., Knecht J., Kröger A. Hydrogenase from Vibrio succinogenes, a nickel protein. FEBS Lett. 1982 Aug 23;145(2):230–234. doi: 10.1016/0014-5793(82)80173-7. [DOI] [PubMed] [Google Scholar]
- Van Dijk C., Mayhew S. G., Grande H. J., Veeger C. Purification and properties of hydrogenase from Megasphaera elsdenii. Eur J Biochem. 1979 Dec 17;102(2):317–330. doi: 10.1111/j.1432-1033.1979.tb04246.x. [DOI] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. Solubilization, purification and properties of particulate hydrogenase from Desulfovibrio vulgaris. J Biochem. 1970 Nov;68(5):649–657. doi: 10.1093/oxfordjournals.jbchem.a129398. [DOI] [PubMed] [Google Scholar]
- van Dijk C., Grande H. J., Mayhew S. G., Veeger C. Properties of the hydrogenase of Megasphaera elsdenii. Eur J Biochem. 1980;107(1):251–261. doi: 10.1111/j.1432-1033.1980.tb04645.x. [DOI] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]


