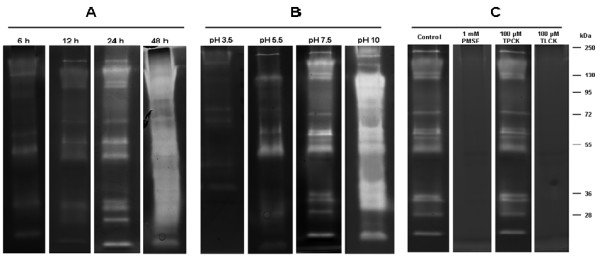
Figure 1.
Time course, influence of pH, and effect of peptidase inhibitors on the proteolytic activity from eggs of Culex quinquefasciatus.(A) For the time course analysis, the proteolytic activity was detected by incubation of SDS-gelatin gels at 37°C in 100 mM Tris–HCl (pH 7.5); (B) the influence of pH on the enzyme activity was evaluated after incubation of the gels at 37°C for 24 h in 100 mM sodium acetate (pH 3.5 or 5.5) or in 100 mM Tris–HCl (pH 7.5 or 10.0); (C) For evaluating the effect of peptidase inhibitors on the peptidase profile, the samples were pre-incubated for 30 min in the presence of each of the following inhibitors: 1 mM PMSF, 100 μM TPCK or 100 μM TLCK. Then, proteolytic activity was detected after incubation of the gels for 24 h at 37°C in 100 mM Tris–HCl (pH 7.5). The control was processed under the same conditions but in the absence of inhibitors. The numbers on the right of each image indicate the apparent molecular masses of the peptidases, expressed in kDa.