Abstract
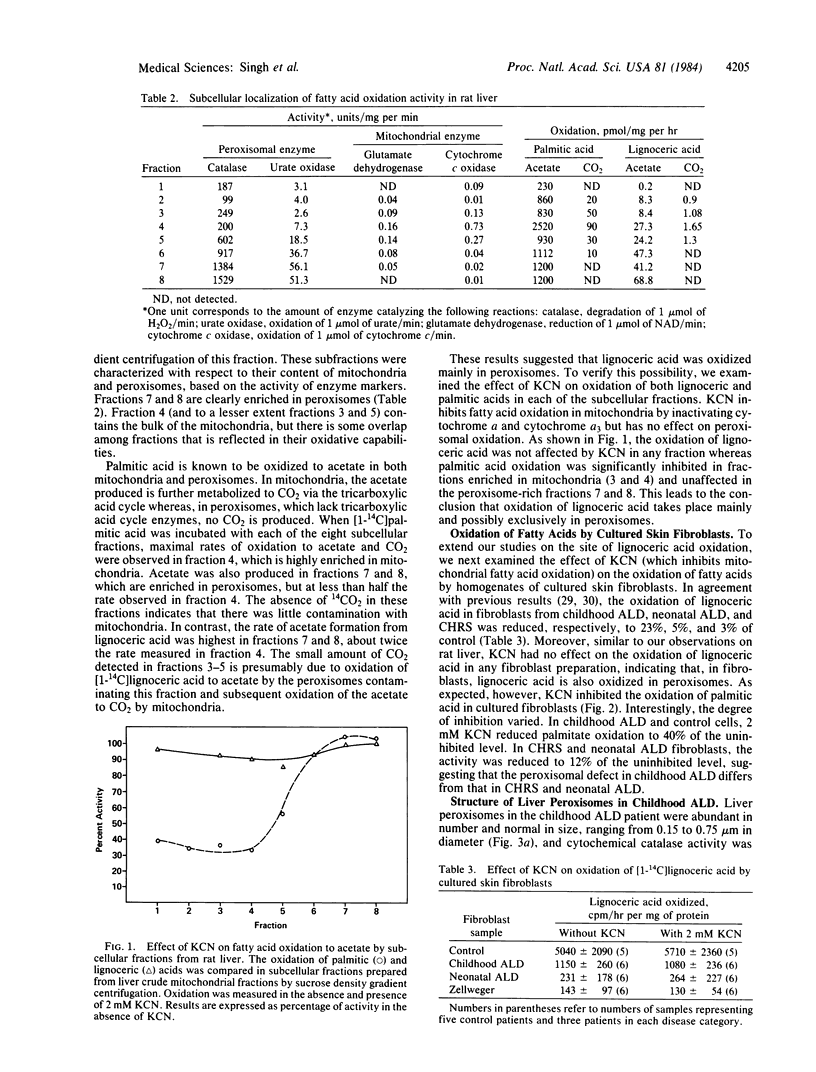
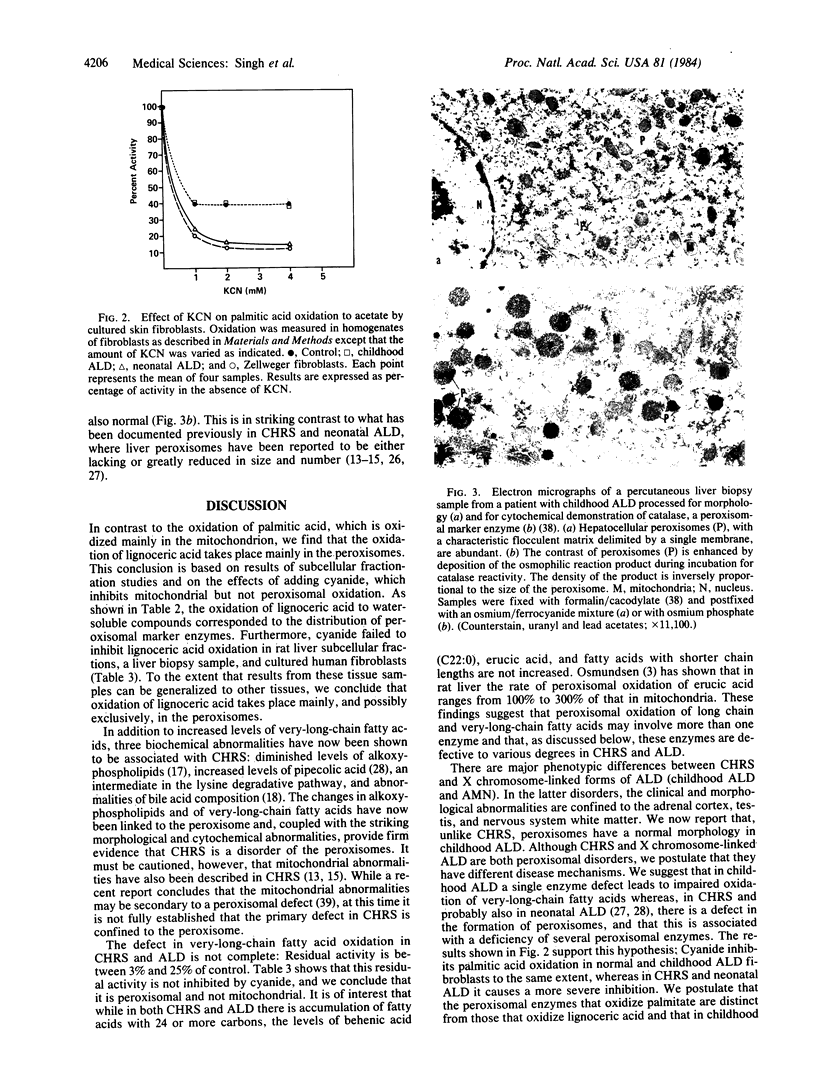
The deficient oxidation and accumulation of very-long-chain fatty acids in the Zellweger cerebro-hepato-renal syndrome (CHRS) and X chromosome-linked adrenoleukodystrophy (ALD), coupled with the observation that peroxisomes are lacking in CHRS, prompted us to investigate the subcellular localization of the catabolism of lignoceric acid (C24:0). Peroxisomal and mitochondrial-rich fractions were separated from rat liver crude mitochondria by sucrose density gradient centrifugation. Enzyme activity for the oxidation of [1-14C]palmitic acid to water-soluble acetate was 2- to 3-fold higher in the mitochondrial than in the peroxisomal-rich fraction whereas [1-14C]lignoceric acid was oxidized at a 2- to 3-fold higher rate in the peroxisomal than in the mitochondrial fraction. Moreover, unlike palmitic acid oxidation, lignoceric acid oxidation was not inhibited by potassium cyanide in either rat liver fractions or human skin cultured fibroblasts, showing that lignoceric acid is mainly and possibly exclusively oxidized in peroxisomes. We also conducted studies to clarify the striking phenotypic differences between CHRS and the childhood form of ALD. In contrast to CHRS, we found normal hepatocellular peroxisomes in the liver biopsy of a childhood ALD patient. In addition, in the presence of potassium cyanide, the oxidation of palmitic acid in cultured skin fibroblasts was inhibited by 62% in control and X chromosome-linked ALD patients compared with 88% in CHRS and neonatal ALD. This differential effect may be related to differences in peroxisomal morphology in those disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMEYER H. U. Zur Messung von Katalase-Aktivitäten. Biochem Z. 1955;327(4):255–258. [PubMed] [Google Scholar]
- Brown F. R., 3rd, McAdams A. J., Cummins J. W., Konkol R., Singh I., Moser A. B., Moser H. W. Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopkins Med J. 1982 Dec;151(6):344–351. [PubMed] [Google Scholar]
- Danks D. M., Tippett P., Adams C., Campbell P. Cerebro-hepato-renal syndrome of Zellweger. A report of eight cases with comments upon the incidence, the liver lesion, and a fault in pipecolic acid metabolism. J Pediatr. 1975 Mar;86(3):382–387. doi: 10.1016/s0022-3476(75)80967-x. [DOI] [PubMed] [Google Scholar]
- Evrard P., Caviness V. S., Jr, Prats-Vinas J., Lyon G. The mechanism of arrest of neuronal migration in the Zellweger malformation: an hypothesis bases upon cytoarchitectonic analysis. Acta Neuropathol. 1978 Feb 20;41(2):109–117. doi: 10.1007/BF00689761. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gilchrist K. W., Gilbert E. F., Shahidi N. T., Opitz J. M. The evaluation of infants with the Zellweger (cerebro-hepato-renal) syndrome. Clin Genet. 1975 May-Jun;7(5):413–416. doi: 10.1111/j.1399-0004.1975.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Goren E., Schaumburg H., Engel W. K., Loriaux L. Adrenomyeloneuropathy: a probable variant of adrenoleukodystrophy. I. Clinical and endocrinologic aspects. Neurology. 1977 Dec;27(12):1107–1113. doi: 10.1212/wnl.27.12.1107. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Bishop J. E. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Szczepanik-VanLeeuwen P., Williams G. C., Grabowski G., Sharp H. L. Defects of bile acid synthesis in Zellweger's syndrome. Science. 1979 Mar 16;203(4385):1107–1108. doi: 10.1126/science.424737. [DOI] [PubMed] [Google Scholar]
- Heymans H. S., Schutgens R. B., Tan R., van den Bosch H., Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature. 1983 Nov 3;306(5938):69–70. doi: 10.1038/306069a0. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Kishimoto Y. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J Biol Chem. 1973 Jun 10;248(11):4123–4130. [PubMed] [Google Scholar]
- Jaffe R., Crumrine P., Hashida Y., Moser H. W. Neonatal adrenoleukodystrophy: clinical, pathologic, and biochemical delineation of a syndrome affecting both males and females. Am J Pathol. 1982 Jul;108(1):100–111. [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Moser H. W., Moser A. B., Axelman J., Sillence D., Norum R. A. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser A. E., Singh I., Brown F. R., 3rd, Solish G. I., Kelley R. I., Benke P. J., Moser H. W. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984 May 3;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- Moser H. W., Moser A. B., Frayer K. K., Chen W., Schulman J. D., O'Neill B. P., Kishimoto Y. Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology. 1981 Oct;31(10):1241–1249. doi: 10.1212/wnl.31.10.1241. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Osmundsen H. Peroxisomal beta-oxidation of long fatty acids: effects of high fat diets. Ann N Y Acad Sci. 1982;386:13–29. doi: 10.1111/j.1749-6632.1982.tb21404.x. [DOI] [PubMed] [Google Scholar]
- Passarge E., McAdams A. J. Cerebro-hepato-renal syndrome. A newly recognized hereditary disorder of multiple congenital defects, including sudanophilic leukodystrophy, cirrhosis of the liver, and polycystic kidneys. J Pediatr. 1967 Nov;71(5):691–702. doi: 10.1016/s0022-3476(67)80205-1. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Gustafsson J. Conversion of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid into cholic acid by rat liver peroxisomes. FEBS Lett. 1980 Dec 1;121(2):345–348. doi: 10.1016/0014-5793(80)80377-2. [DOI] [PubMed] [Google Scholar]
- Pfeifer U., Sandhage K. Licht- und Elektronenmikroskopische Leberbefunde beim Cerebro-Hepato-Renalen Syndrom nach Zellweger (Peroxisomen-Defizienz). Virchows Arch A Pathol Anat Histol. 1979 Oct;384(3):269–284. doi: 10.1007/BF00428229. [DOI] [PubMed] [Google Scholar]
- Roels F., Goldfischer S. Cytochemistry of human catalase. The demonstration of hepatic and renal peroxisomes by a high temperature procedure. J Histochem Cytochem. 1979 Nov;27(11):1471–1477. doi: 10.1177/27.11.92501. [DOI] [PubMed] [Google Scholar]
- Schaumburg H. H., Powers J. M., Raine C. S., Suzuki K., Richardson E. P., Jr Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975 Sep;32(9):577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- Singh I., Kishimoto Y. Alpha hydroxylation of lignoceric acid in brain. Subcellular localization of alpha hydroxylation and the requirement for heat-stable and heat-labile factors and sphingosine. J Biol Chem. 1979 Aug 25;254(16):7698–7704. [PubMed] [Google Scholar]
- Singh I., Moser A. E., Moser H. W., Kishimoto Y. Adrenoleukodystrophy: impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr Res. 1984 Mar;18(3):286–290. doi: 10.1203/00006450-198403000-00016. [DOI] [PubMed] [Google Scholar]
- Singh I., Moser H. W., Moser A. B., Kishimoto Y. Adrenoleukodystrophy: impaired oxidation of long chain fatty acids in cultured skin fibroblasts an adrenal cortex. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1223–1229. doi: 10.1016/s0006-291x(81)80142-8. [DOI] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. The degradation of urate in liver peroxisomes. Association of allantoinase with allantoicase in amphibian liver but not in fish and invertebrate liver. J Biol Chem. 1983 Apr 25;258(8):4762–4764. [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Trijbels J. M., Berden J. A., Monnens L. A., Willems J. L., Janssen A. J., Schutgens R. B., van den Broek-Van Essen M. Biochemical studies in the liver and muscle of patients with Zellweger syndrome. Pediatr Res. 1983 Jun;17(6):514–517. doi: 10.1203/00006450-198306000-00018. [DOI] [PubMed] [Google Scholar]
- Versmold H. T., Bremer H. J., Herzog V., Siegel G., Bassewitz D. B., Irle U., Voss H., Lombeck I., Brauser B. A metabolic disorder similar to Zellweger syndrome with hepatic acatalasia and absence of peroxisomes, altered content and redox state of cytochromes, and infantile cirrhosis with hemosiderosis. Eur J Pediatr. 1977 Mar 18;124(4):261–275. doi: 10.1007/BF00441934. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Adams R. D. Cerebro-hepato-renal syndrome of Zellweger: an inherited disorder of neuronal migration. Acta Neuropathol. 1972;20(3):175–198. doi: 10.1007/BF00686900. [DOI] [PubMed] [Google Scholar]




