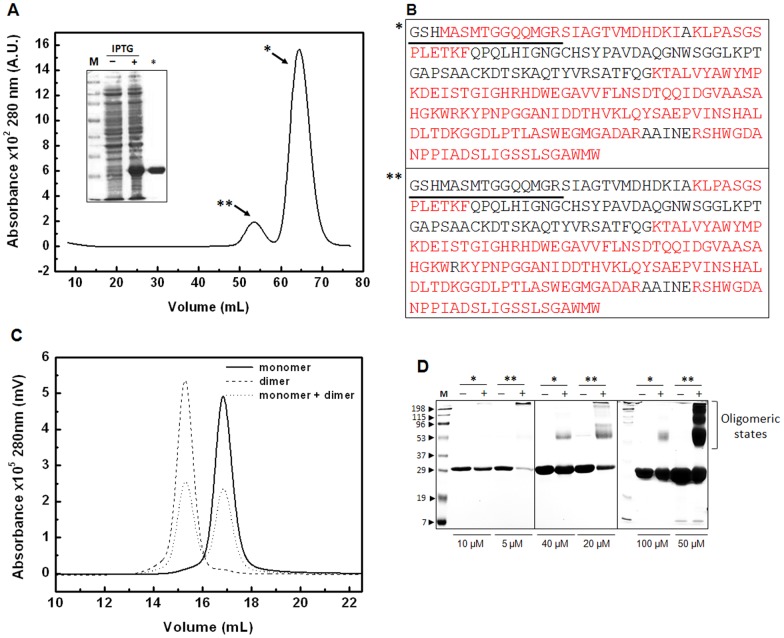
Figure 1. MpNep2 dimers co-exist with monomers.
A. Preparative size-exclusion chromatography performed on Sephacryl S100 16/60 showed eluted peaks that correspond to MpNep2 monomer (*) and MpNep2 dimer (**). Inset shows a representative 15% SDS-PAGE of the purification protocol. (−) lane shows protein extract before IPTG induction and (+) lane show protein extract 16 h after induction with 1 mM IPTG. (*) lane shows MpNep2 monomer after purification. B. Protein coverage by electron-spray mass spectrometry is shown for MpNep2 monomer (*) and dimer (**). The entire MpNep2 sequence expressed by pET28a vector is shown. Common tryptic peptides are highlighted in red, cloning artifacts are underlined, and non-detected peptides are in black. C. Analytical size-exclusion chromatography of MpNep2 monomer (solid line), dimer (dashed line) and a mixture with equal amounts of each (dotted line) was performed on Superdex 200. Preparative and analytical chromatography at 0.7 mL.min−1 were monitored at 280 nm. D. 15% SDS-PAGE following glutaraldehyde cross-linking of MpNep2 monomer (*) and dimer (**) at different concentrations. (−) lanes show controls without glutaraldehyde and (+) lanes show cross-linked proteins after 5 min with 0.1% glutaraldehyde at 37°C. Cross-linking reactions were performed separately with equal amounts between monomers and dimers. 10, 40 and 100 μM were used for monomers and 5, 20 and 50 μM were used for dimers.