Fig. 4.
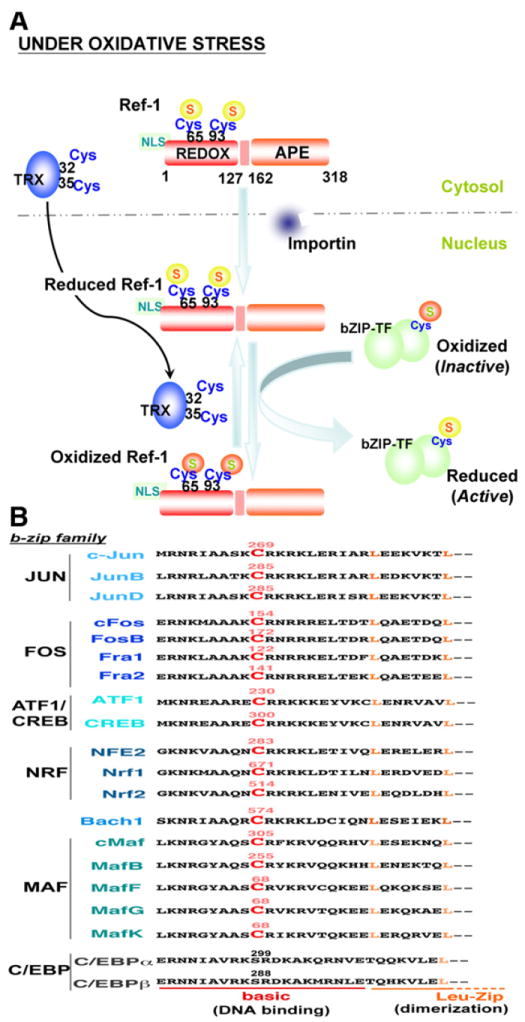
Ref1-mediated redox cell signaling. A) The human Ref-1N-terminal region consists of the redox domain (REDOX; residues 1–127) and a 20 amino acid nuclear localization sequence (NLS); the C-terminal region contains the apurinic/apyrimidinic endonuclease domain (APE; residues 162–318). Cysteine-65 and -93 are major redox active sites. Under oxidative stress, Ref-1 translocates into the nucleus by NLS-importin-dependent or -independent pathways where Ref-1 regulates the activity of b-zip transcription factors (bZIP-TF) by redox mechanisms. Oxidized Ref-1 is subject to redox regulation by nuclear translocated thioredoxin (TRX) through the TRX catalytic center (Cys-32 and -35). B) The conserved redox-active cysteine of various human b-zip transcription factors is indicated in red color and labeled with the residue number. In addition to the b-zip family, other transcription factors such as p53, NFκB, and HIF-1α are also regulated by Ref-1 [56].
