Abstract
BAX, the BCL-2-associated X protein, is a cardinal pro-apoptotic member of the BCL-2 family, which regulates the critical balance between cellular life and death. Because so many medical conditions can be categorized as diseases of either too many or too few cells, dissecting the biochemistry of BCL-2 family proteins and developing pharmacologic strategies to target them have become high priority scientific objectives. Here, we focus on BAX, a latent, cytosolic, and monomeric protein that transforms into a lethal mitochondrial oligomer in response to cellular stress. New insights into the structural location of BAX's “on” switch, and the multi-step conformational changes that ensue upon BAX activation, are providing fresh opportunities to modulate BAX for potential benefit in human diseases characterized by pathologic cell survival or unwanted cellular demise.
BCL-2 and BAX: the yin and yang of apoptosis
The founding member of the BCL-2 family was “born” in 1984, when a cell line derived from a pediatric patient with acute lymphoblastic leukemia (ALL) was found to contain, among its defects, an oncogenic translocation that fused the immunoglobulin heavy chain locus on chromosome 14 with the B-cell lymphoma 2 gene (BCL2) of unknown function on chromosome 181. The t(14;18) breakpoint, also identified in follicular lymphoma, was cloned and characterized2–4, and the gene product found to promote cell survival5, 6. In 1993, the first BCL-2 interaction partner, BCL-2-associated X protein (BAX), was identified as a highly homologous protein that could self-associate or heterodimerize with BCL-2, but in striking contrast to BCL-2, promoted, rather than blocked, cell death after a stress stimulus7. With the discovery of BAX, the rheostat model of apoptosis was formulated, positioning BAX and BCL-2 as the yin and yang whose relative levels dictated the balance between cellular life and death7 (Figure 1).
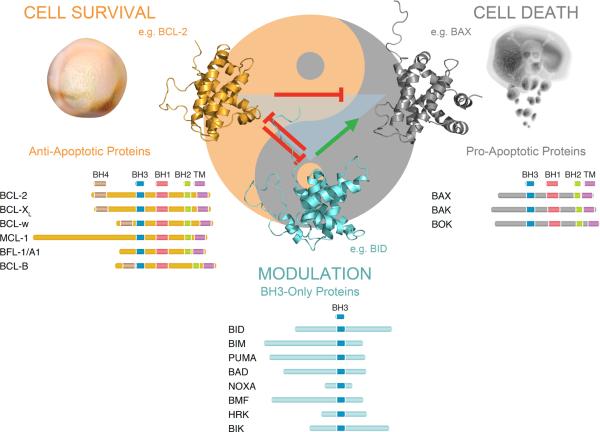
Figure 1. The BCL-2 Family of Anti- and Pro-Apoptotic Proteins.
The BCL-2 family comprises three classes of apoptotic proteins, which participate in an interaction network that controls the critical balance between cellular life and death. Multi-BCL-2 homology (BH) domain anti-apoptotic proteins promote cell survival by heterodimerizing with and blocking the oligomerization of multi-BH domain pro-apoptotic proteins and by neutralizing BH3-only proteins. The proapoptotic BH3-only subclass modulates the multi-BH domain proteins through engagement by their single conserved BH3 domain.
Walensky and Gavathiotis Figure 01
With the prototype life and death members defined, the BCL-2 family rapidly expanded to include anti-apoptotic MCL-18, BCL-XL9, BFL-1/A110, 11, BCL-w12, and BCL-B13, and pro-apoptotic BAK14–16 and BOK17 (Figure 1). BCL-2 family membership was originally defined by the presence of at least three of four BCL-2 homology (BH) domains. The subcellular site of BCL-2's pro-survival activity was first linked to mitochondria in 199018, with ultrastructural analysis revealing the outer mitochondrial membrane as the predominant intracellular destination of BCL-219. Whereas immunohistochemical analysis of pro-apoptotic BAK expression likewise demonstrated a punctuate pattern consistent with a constitutive organeller localization20, BAX was found to have a diffuse cytosolic distribution during homeostatic conditions, but then moved to the mitochondria upon induction of apoptosis21. Once the release of mitochondrial factors such as cytochrome c into the cytosol was determined in 1996 to be a key step of the apoptotic signaling pathway22, it soon became clear that the mitochondrial-localized BCL-2 family members dictated the life-death balance by regulating mitochondrial integrity23, 24 (Box 1). Indeed, the explicit mechanisms and protein interactions that fully account for the mitochondrial guardian and executioner functions of BCL-2 family proteins remain active areas of scientific investigation to this day.
To BH3 or not to BH3
A comprehensive deletional analysis localized both the killing activity and BCL-XL-binding activity of pro-apoptotic BAK to a discrete, conserved protein subdomain dubbed BCL-2 homology domain 3 (BH3)25. The same region in BAX was sufficient for binding and blocking the adenovirus BCL-2 homologue E1B 19K26. In 1997, the structural basis for the death-defying interaction between the BAK BH3 domain and BCL-XL was defined as a complex between an amphipathic BH3 alpha-helix and a surface groove on BCL-XL27. That year, the BH3 domains of BAX and BAK, including peptides corresponding to shorter core sequences within the BH3 domains, were found to induce apoptosis in a cell-free system28. Whereas the BH3 domain emerged as a key interaction motif of the apoptotic process, its relative roles in binding anti-apoptotic grooves and permeabilizing mitochondrial membranes remained unclear. A subsequent BAX BH3 domain mutagenesis study dissociated the homo- and heterodimerization activity of BAX with its capacity to self-associate in mitochondrial membranes and induce dysfunction, suggesting that BAX's killing activity derives from an alternative (and still unknown) intramembranous conformation29.
Yet another layer of complexity emerged when proteins such as BAD30, BIK31, 32, BID33, BIM34, and others were discovered, classified as pro-apoptotic, but only contained a conserved BH3 domain (Figure 1). These “BH3-only” proteins expanded the web of BCL-2 family interactions, leading to different theories of just how this new class of proteins modulated the activities of “multidomain” members such as BAX and BCL-2 (Figure 2). It is widely agreed upon that exposed BH3 domains are pro-apoptotic unless bound and neutralized by the surface grooves of anti-apoptotic proteins. For example, if a multidomain anti-apoptotic protein has captured the exposed BH3 domain of a multidomain pro-apoptotic protein, deployment of BH3-only proteins can “inhibit the inhibitors” of cell death, functionally derepressing BAX/BAK and provoking apoptosis35 (Figure 2). Likewise, unbound anti-apoptotic proteins can be targeted by BH3-only proteins to decrease the “anti-apoptotic reserve” and lower the apoptotic threshold36, 37. Somewhat more controversial is whether select BH3-only proteins can activate mitochondrial apoptosis through direct interaction with BAX/BAK and the relative role of this mechanism in apoptosis regulation33, 38 (Figure 2). Here, we review the latest insights into the direct activation mechanism of pro-apoptotic BAX, highlighting how the relationship between structure, biochemistry, and function drive this fundamental and dynamic apoptotic process.
Figure 2. BCL-2 Family Signaling Dynamics.
Mitochondrial apoptosis is regulated by the BH3 domain interactions of apoptotic proteins. In response to stress stimuli, BH3-only proteins promote BAX activation through direct and indirect mechanisms. Select BH3-only proteins bind directly to BAX and trigger its conformational activation, leading to mitochondrial translocation, oligomerization, and permeabilization of the mitochondrial outer membrane. BH3-only proteins also promote BAX oligomerization indirectly by targeting the BH3-binding pocket of anti-apoptotic proteins, releasing BH3-only direct activators and conformationally-active forms of BAX that were sequestered in heterodimeric complex. Conversely, anti-apoptotic proteins prevent BAX-mediated mitochondrial apoptosis by impounding the pro-apoptotic BH3 domains of BH3-only proteins and conformationally-active BAX in a surface groove, effectively suppressing BH3-only direct BAX activation and BAX oligomerization, respectively.
Walensky and Gavathiotis Figure 02
Turning BAX on
One of the key differences between BAX and BAK is that BAX resides predominantly in the cytosol, whereas BAK is localized to the mitochondrial outer membrane. How BAK is maintained in an inactive state under homeostatic conditions is a matter of debate and is believed to involve interactions with anti-apoptotic proteins35, voltage-dependent anion channel 2 (VDAC2)39, 40, and/or yet unknown mechanisms. The picture is somewhat clearer for BAX as it resides inactive in the cytosol, presumably auto-inhibited through sequestration of its hydrophobic surfaces at the protein core, although other negative regulatory proteins and dynamic trafficking mechanisms may be involved41, 42. Indeed, the NMR structure of monomeric BAX demonstrated that the hydrophobic surface of its BH3 domain faces inward and the groove corresponding to the BH3-binding pocket found in anti-apoptotic proteins is plugged by its own C-terminal helix43. How then is BAX turned on in the context of cellular stress? The first notion that BAX could be directly activated by a BH3 domain derived from the discovery of BID, which was identified as a result of its dual interactions with BCL-2 and BAX33. Furthermore, discrete mutations in the BID BH3 domain abrogated the BCL-2 interaction but preserved its capacity to bind BAX and induce apoptosis33. Caspase-8-cleaved tBID44–46 and peptides corresponding to the BH3 domains of BID and BIM47, 48 activated BAX-mediated membrane pore formation in liposomal systems. The use of this reductionist assay highlighted that select BH3 domains could directly activate BAX in the absence of other cellular factors.
As the biochemical evidence for BH3-induced direct BAX activation mounted, the explicit nature of the binding interactions remained elusive. Whereas ample in vitro binding data using synthetic BH3 domain peptides demonstrated their capacity to directly and differentially engage recombinant anti-apoptotic targets (albeit the C-terminally truncated versions of these proteins with surface-exposed BH3-binding pockets)49, 50, appreciable binding to full-length BAX was not detected, even for BID and BIM BH3 peptides35, 51. Based on these binding data, it was plausible to conclude that BH3–BAX interactions simply did not occur, or that there was something different about the nature of BH3 interactions with proapoptotic proteins relative to anti-apoptotic proteins. One basic difference is that BH3–anti-apoptotic protein interactions are static, and thus amenable to conventional structural and biochemical analyses, whereas putative interactions with BAX were proposed to be dynamic or “hit and run”38, 52, 53. In the latter case, transient BH3–BAX binding interactions would induce a global conformational change and transform BAX from an inactive cytosolic protein into a mitochondrial homo-oligomeric pore, a process less amenable to protein complex capture and analysis using traditional methods. Another potentially confounding issue was that the synthetic BH3 peptides being applied in binding studies were predominantly unstructured in solution37, whereas BH3 domains are α-helical in the context of the native proteins and/or when bound to their apoptotic targets27, 43, 54, 55. To restore α-helical structure to the unfolded peptides, we generated Stabilized Alpha-Helix of BCL-2 domains (“SAHBs”), in which non-natural amino acids containing olefinic tethers are installed at (i, i+4) or (i, i+7) positions and then crosslinked by ruthenium-catalyzed olefin metathesis to yield a structurally-reinforcing hydrocarbon “staple”56, 57. SAHBs modeled after the BID, BIM, and BAD BH3 domains bound to anti-apoptotic targets with high affinity and in a sequence-dependent manner, and when employed in BAX-binding analyses51, 56, BIM and BID SAHBs, but not their binding interface point mutants or BAD SAHB, displayed measurable, low nanomolar binding interactions with BAX for the first time51. With evidence of a direct binding interaction that functionally activated BAX in hand, the capacity to characterize the complex using structural methods was potentially in reach.
Initial NMR analyses of 15N-BAX upon BIM SAHB titration highlighted the catch-22 of trying to study the structure of, literally, a moving target. Because ligand binding triggered rapid BAX oligomerization, precluding the structural analysis, the composition of BIM SAHB was adjusted to weaken its binding activity in an effort to slow down the activation process. Ultimately, removal of two C-terminal residues and addition of one native N-terminal residue accomplished this goal. The NMR experiments identified chemical shift changes in a discrete subset of BAX residues that colocalized to a surface groove formed by the confluence of α-helices 1 and 6 at the N-terminal face of the protein58. Whereas the topography of the BIM SAHB binding site shared striking similarities with the BH3-binding site of anti-apoptotic proteins (Figure 3A–B), including a nearly identical orientation of key charged and hydrophilic residues at the perimeter of the hydrophobic groove, the interaction site on BAX was on the opposite side of the protein from the canonical BH3-binding groove, which in BAX is tightly engaged by its own C-terminal α-9 helix (Figure 3C). Because the complex was insufficiently stable to solve the solution structure by conventional NMR, we generated spin-labeled derivatives of BIM SAHB for paramagnetic relaxation enhancement (PRE) NMR analyses. This methodology provided critical distance information between the BIM BH3 helix and BAX, enabling the calculation of a model structure that in turn formed the basis for mutagenesis studies to functionally validate the direct interaction in biochemical and cellular assays. Mutagenesis of binding interface residues on BIM BH3 and BAX predicted to be important by the calculated model structure indeed abrogated or impaired functional BAX activation in vitro and in cells. BIM SAHB constructs in which the staple was differentially localized along the non-interacting surface of the BH3 helix induced BAX activation with similar potency and specificity, whereas a staple placed at the interaction surface disrupted ligand binding and functional activity, as anticipated58. Importantly, a BAD SAHB construct containing the identical staple position as the prototype BIM SAHB neither bound to BAX by NMR analysis nor triggered BAX activation, further highlighting the sequence-dependent activity of BIM SAHB. Thus, we concluded that the “on switch” for selective, BH3-induced direct BAX activation resides at a novel trigger site formed by α1 and α6 residues on the N-terminal surface of the death protein.
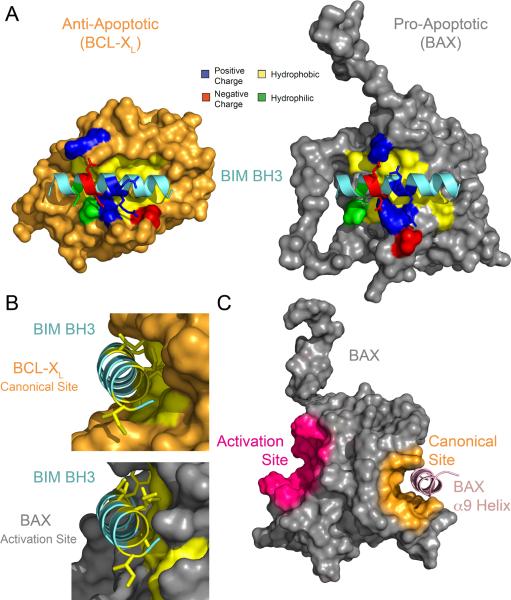
Figure 3. The BH3 Binding Sites of Apoptotic Proteins.
The BIM BH3 interaction site on pro-apoptotic BAX is topographically similar but geographically distinct from that on anti-apoptotic BCL-XL. (A) The BH3 interaction sites at the C-terminal face of BCL-XL and N-terminal face of BAX share a similar orientation of polar, positively charged, and negatively charged residues that engage complementary residues of the BIM BH3 α-helix. (B) The bulk of the BIM BH3 binding interfaces with BCL-XL and BAX comprise extensive contacts between the hydrophobic face of the α-helix and the hydrophobic cleft formed at the protein surface by a confluence of residues from BCL-XL α-helices 2, 4, 5, 7, and 8, and BAX α-helices 1 and 6. (C) In the inactive, monomeric form of BAX, the canonical BH3 binding pocket is occupied by BAX's C-terminal α9 helix. By contrast, the surface groove of the BAX activation site, located on the opposite side of the protein, is accessible for BH3 triggering.
Walensky and Gavathiotis Figure 03
Interestingly, this unexpected result provided a potential explanation for why unfolded BIM BH3 peptides readily bound to the surface groove of C-terminally truncated anti-apoptotic proteins but not to full-length BAX: the binding sites were different (Box 2). In addition to the two sites being localized to opposite sides of the BCL-2 family proteins, the anti-apoptotic pocket is notably deeper than that of the BAX trigger site, facilitating α-helical folding by induced fit in the case of anti-apoptotic binding, but necessitating a greater degree of prefolding in the case of BAX binding. In the native context, the structure of the BH3 domain is dictated both by the protein in which it is embedded and interactions with the protein target. However, confronted by sequence-compatible binding pockets in the in vitro setting, an unfolded peptide would favor binding to a deeper pocket, whereas a structurally-stabilized α-helix could readily engage both surfaces. With BAX's elusive “on switch” revealed, how does flipping the switch trigger the biochemical transformation of BAX from innocent bystander to lethal weapon?
A Global Conformational Change from N- to C-terminus
Soon after the killing activity of BAX was discovered7, it became clear that to accelerate programmed cell death BAX underwent both a conformational change from monomer to pore-forming oligomer59, 60 and a subcellular localization change from cytosol to mitochondria21, 61. Indeed, structural and functional alterations in BAX could be elicited by a variety of biophysical stimuli, including exposure to discrete classes of detergents62, changes in pH63, 64, heat65, and engagement by select BH3-only proteins, such as tBID66. The requirement for a global conformational change was further supported by the solution structure of monomeric BAX, which showed the hydrophobic surfaces, presumably required for membrane-targeting and intramembrane pore-forming interactions, buried within the protein core43. Given what is known about the structure of inactive BAX43, 58 and its functional mitotoxic endpoint, how does a physiologic trigger induce the total conformational reorganization of BAX and what are the structural features of BAX-in-motion?
The identification of a BH3 trigger site on BAX at its N-terminal face is consistent with a series of prior observations implicating the BAX N-terminus in the regulation of BAX activation. First, a conformation-specific BAX antibody recognizes amino acids 12–24 of the N-terminus only in the activated form of BAX, suggesting that activation is linked to exposure of this N-terminal epitope67. Interestingly, we noted that our calculated model structures of the BIM BH3–BAX interaction favored a conformational change in the loop between BAX α-helices 1 and 2. Whereas in the unbound monomer, the α1–α2 loop engaged in non-covalent interactions with residues of the α1/α6 surface (“closed” position)43, in the liganded structure, the loop was displaced by the BIM BH3 helix (“open” position)68. Treatment of recombinant BAX with BIM SAHB dose-responsively exposed the 6A7 epitope, but when the α1–α2 loop was locked into its closed conformation by an installed disulfide tether, BIM SAHB binding was no longer capable of triggering 6A7 exposure or BAX activation68. Reduction of the disulfide bond completely restored ligand-induced BAX activation, implicating α1–α2 loop displacement as the initiating conformational change of the BAX activation pathway (Figure 4A).
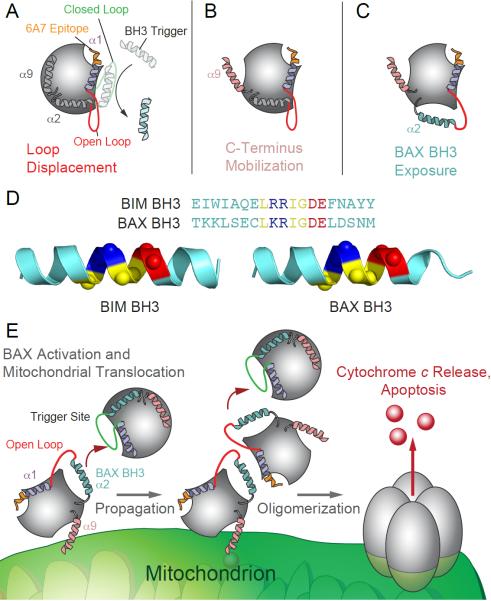
Figure 4. The BH3-triggered Direct BAX Activation Pathway.
BAX activation is triggered by BIM BH3 and self-propagated by BAX BH3 through direct engagement of the α1/α6 binding site. (A) The triggering interaction between BIM BH3 and BAX unleashes a series of structural changes, starting with displacement of the α1-α2 loop, converting it from a closed (green) to an open (red) position, and resultant exposure of the 6A7 activation epitope (orange). The BIM BH3 binding interaction and initiating conformational change at the N-terminal face induces (B) the mobilization of BAX's C-terminal mitochondrial membrane insertion helix (pink) and (C) exposure of the hydrophobic interaction surface of the BAX BH3 domain (cyan). (D) Sequence and structural alignments of the BIM and BAX BH3 domains demonstrate striking amino acid identity and orientation of the core BH3 sequences that engage the BAX trigger site. (E) Consequently, once triggered, BAX propagates its activation through interactions between the exposed BAX BH3 domain of fully activated monomers and the α1/α6 binding site of inactive monomers. BAX assembles into a structurally undefined homo-oligomeric pore that promotes apoptosis by releasing mitochondrial factors such as cytochrome c.
Walensky and Gavathiotis Figure 04
Importantly, a variety of studies have documented a regulatory relationship between the N-terminus of BAX and its C-terminus, which contains the nascent α9 transmembrane domain for mitochondrial insertion69–73. Mutagenesis of key proline residues at the N- and C-terminus caused significant alterations in BAX function, presumably by impacting the structure and mobility of these key regulatory domains69, 72–75. Likewise, we have found that treatment of BAX with increasing concentrations of BIM SAHB caused dose-responsive and allosteric chemical shift changes in a series of α9 residues, as assessed by NMR68. If α9 is locked into its binding pocket (the canonical BH3 binding groove) by installation of a disulfide tether, the ligand-induced allosteric changes in α9 are blocked, as are BIM SAHB-induced BAX translocation to mitochondria and BAX-mediated cytochrome c release68. Reduction of the disulfide tether completely restored ligand-triggered BAX translocation and cytochrome c release, highlighting that engagement of BIM SAHB at the BAX N-terminus impacts the structure and function of the BAX C-terminus (Figure 4B).
Exposure of the BAX death domain: Capture or Consequence
The second alpha-helix of BAX is its BH3 death domain, required for both killing activity and anti-apoptotic protein interaction. Importantly, the functional interaction surface of this amphipathic helix is the hydrophobic one29, which is buried within the core of the inactive monomer43. Thus, in order for BAX to manifest its killer instinct or become captured by the BH3-binding pocket of anti-apoptotic proteins, the hydrophobic face of its BH3 domain must also emerge as a consequence of the global conformational change that ensues upon BAX activation. Whereas it proved difficult to capture the exposure of BAX BH3 upon stimulation of BAX with BIM SAHB, likely due to rapid burying of the hydrophobic surface in oligomeric species, trapping BAX in a conformational intermediate through disulfide constraint of α9 revealed dose-responsive BIM SAHB-induced exposure of BAX BH3 as detected both by NMR and anti-BH3 immunoprecipitation68. Similar results were obtained using a C-terminal proline mutant of BAX that exhibits defective mitochondrial translocation and cytochrome c release due to α9 dysfunction68. Thus, BH3-induced direct BAX activation launches the effector surface of pro-apoptotic BAX (Figure 4C).
The functional importance of the hydrophobic surface of the BAX BH3 helix has been documented both by in vitro29 and in vivo76, 77 mutational analyses. Whereas the molecular details of how BAX BH3 engages anti-apoptotic binding pockets are well defined78, the protein interaction-based mechanism by which BAX BH3 exerts its killing activity remained unknown. The original BAX BH3 mutagenesis study dissociated BAX's BCL-2 binding, BAX binding, and killing activities29, suggesting that discrete molecular features of BAX BH3 could drive these physiologic activities independently. A more recent study confirmed that single point mutagenesis of BAX BH3 could impair anti-apoptotic interaction yet preserve killing functionality78, again highlighting that the BAX BH3 interactions driving BAX-mediated apoptosis may be distinct. Once activated, BAX can propagate its own activation if its BH3 domain remains unopposed79, but the molecular nature of these autoactivating interactions remained unknown.
Having defined the structural features of the triggering interaction between BIM BH3 and BAX, we recognized that BAX BH3 shared striking sequence identity to BIM BH3 within the core BH3 binding cassette (Figure 4D). To test the hypothesis that, once triggered, the BAX BH3 domain itself could assume the role of a triggering BH3 helix, we generated BAX SAHBs for NMR and biochemical analyses. We found that BAX SAHB, like BIM SAHB, engaged BAX at the N-terminal trigger site and induced sequence-specific BAX oligomerization and BAX-mediated cytochrome c release68. Importantly, single point mutagenesis of key interacting residues of BAX BH3 and the BAX trigger site impaired ligand-induced and heat-induced BAX activation, with functional activity fully restored by complementary mutagenesis68. These data implicated the interaction between BAX BH3 and the trigger site as the mechanism for BAX self-propagation, a key step along the path to BAX-mediated mitochondrial outer membrane permeabilization and apoptosis (Figure 4E).
Lancing the Mitochondrial Outer Membrane
The killing activity of BAX and BAK has been attributed to their capacity to induce permeabilization of the mitochondrial outer membrane80, releasing apoptogenic factors such as cytochrome c and SMAC/Diablo)22, 23, 81. High molecular weight oligomers of BAX are found in the mitochondrial membrane of apoptotic cells, and BAX has inherent pore-forming activity48, 60, 82, 83. Direct imaging of membrane-embedded BAX by atomic force microscopy documented its capacity to form large toroidal pores84. Although a lack of structural information has hampered efforts to fully characterize the protein-protein and protein-lipid interactions of a functional BAX pore, biochemical studies have provided clues as to how membrane insertion domains and self-associating subunits may combine to form an oligomerization unit that functionally pierces the mitochondrial outer membrane.
Models for BAX and BAK oligomerization have emerged based on protein crosslinking analyses that take advantage of installed cysteines or photo-crosslinking probes. Two interaction surfaces have been captured: one between the BH3-binding face of homodimers (BH3 in groove) and the other at an N-terminal interface that comprises α685–87. Based on these data, an oligomer of dimers model for BAX/BAK-mediated mitochondrial outer membrane permeabilization has been proposed85, 87. It remains unclear just how these interaction surfaces, as represented in the solution structures of BCL-2 proteins, are altered to yield an intramembrane oligomeric pore. Indeed, key hydrophobic helices and interfaces of BAX/BAK, buried within the core of the inactive monomer, are likely to undergo dramatic reorganization upon lipid bilayer engagement and poration.
BAX alpha helices 1, 5, and 6, in addition to the C-terminal (α9) transmembrane domain, are believed to be the membrane insertion fragments of a functional BAX pore88. Indeed, helices 5 and 6 of BAX's hydrophobic hairpin can independently form pores in lipid membranes89, 90. An elegant deletion and mutational analysis mapped the minimal essential oligomerization-competent subunit of BAX to helices 2 (BH3), 4, and 5, correlating the capacity to oligomerize with BAX-dependent apoptotic activity91. Whereas BH3 engagement may be required for catalyzing or nucleating oligomerization68, 85, 87, 92, the explicit structure of membrane-embedded BAX and how its homo-oligomeric interactions give rise to a functional pore that incorporates the membrane-spanning helices remains an active area of investigation. The contribution of lipids and potentially other protein components to the kinetics, structure, and functionality of pore assembly represent yet additional layers of physiologic complexity48, 93, 94.
Targeting BAX for Therapeutic Benefit
Given their critical roles as adjudicators of the life/death decision, BCL-2 family proteins have become ripe targets for drug development. Anti-apoptotic proteins have been implicated as pathogenic and chemoresistance factors in a host of human cancers, inspiring the development of inhibitors to neutralize the survival advantage of cancer cells56, 95–98. Although inactivating mutations in BAX have been identified in select cancers76, 99, pathologic mass production of BCL-2 family survival proteins is more common. A cancer cell can achieve a lock on survival by overexpressing one anti-apoptotic allele, compared to the more burdensome task of eliminating all four genetic alleles of BAX and BAK to avoid apoptosis. As a result, the majority of cancer cells contain functional but suppressed BAX and BAK, an antidote that lies within. Indeed, BAX is a key effector of chemotherapy-induced cancer cell apoptosis100. The identification of a novel binding site capable of triggering BAX activation upon direct ligand engagement has opened the door to a new therapeutic strategy for activating apoptosis by deploying cytosolic BAX for mitochondrial assault. The key structural changes that catalyze the transformation of BAX from its inactive to lethal form provide a variety of control points for stimulating the activation process, including dislodging the α1– α2 loop, α9 helix, and BH3 domain to expose BAX's hydrophobic core. Importantly, small molecule inhibitors of anti-apoptotic targets, which are believed to activate BAX indirectly through derepression and/or by displacement of direct activator BH3-only proteins, are exhibiting a therapeutic window in vivo101, 102 (Box 3). Whereas unstressed cells can have sufficient anti-apoptotic reserve to constitutively bind and sequester activated BAX, diseased cells whose anti-apoptotic reserve is taxed by tonic death signaling might be unable to withstand further BAX-mediated mitochondrial assault. Thus, the development and application of indirect and direct BAX activators holds promise to expand the arsenal of targeted therapies for cancer.
On the flip side, targeted inhibition of BAX may avert premature or unwanted cellular demise. Genetic deletion of Bax promotes the survival of discrete cell types during homeostatic and stress conditions. Bax−/− mice display lymphoid hyperplasia103, neuronal salvage in the face of trophic factor deprivation104, prolonged ovarian lifespan owing to excess primordial follicles105, enhanced oligodendrocyte survival after spinal cord injury106, protection from myocardial and hepatic ischemia-reperfusion injury107, 108, reduced myocardial infarct size109, and preservation of photoreceptor cells after retinal detachment110. These examples highlight the potential for pharmacologic inhibition of BAX to preserve or extend the survival of cells subjected to hypoxic, oxidative, radiation-induced, age-related, toxic, or other stresses. The development of lymphoid hyperplasia in Bax−/− mice suggests that temporary BAX blockade, such as in the acute setting of heart attack or stroke, may provide the most suitable therapeutic window, as opposed to chronic use that could potentially promote aberrant cell survival. Two molecular inhibitors of the BAX channel have been reported111, 112, with one demonstrating neuroprotective activity in a mouse model of brain ischemia111. The multistep conformational changes triggered by direct BAX activation may likewise serve as potential targets for BAX inhibition, with effective agents blocking access to the trigger site or restraining the essential movements of the α1–α2 loop, BH3 domain, or C-terminal helix68.
Concluding Remarks
BAX is one of the essential gatekeepers of mitochondrial apoptosis80. A dynamic protein, it transforms from a harmless cytosolic protein into a toxic mitochondrial pore. Much like the phone booth conversion of Clark Kent into Superman, the biochemical and structural changes along the BAX activation pathway are rapid, elusive, and ultimately cloaked (within the mitochondrial outer membrane). However, nearly two decades of BAX research is culminating in the production of a motion picture that features the structural and functional life cycle of this death protein. Although much remains to be discovered and reconciled, the promise of this research is new therapeutic modalities to activate or inhibit BAX so that the homeostatic balance between new and dying cells can be restored.
BOX 1. BCL-2 Family Regulation of Mitochondrial Apoptosis.
The BCL-2 family of apoptosis regulators comprises three protein subgroups (Figure 1). The anti-apoptotic proteins such as BCL-2 contain up to four BCL-2 homology (BH) domains and promote cell survival by binding and blocking the pro-apoptotic proteins, which are divided into two classes. Like the anti-apoptotic proteins, BAX and BAK contain multiple BH domains. A second class of proapoptotic proteins only share homology in the BH3 domain, and were thus dubbed “BH3-only” proteins. BH3-only proteins are situated throughout the cell and subject to diverse modes of regulation, such as phosphorylation, transcriptional upregulation, ubiquitylation, and enzymatic cleavage. In response to pro-apoptotic stimuli from outside or inside the cell, these messenger proteins are deployed to deliver stress signals to the multidomain anti-apoptotic and proapoptotic proteins, which integrate this information at the mitochondrial outer membrane to arrive at a life/death decision (Figure 2). If the BH3-only signals and activated forms of BAX/BAK can be blocked by anti-apoptotic proteins - that is, if there is a sufficient “anti-apoptotic reserve” - then the death signal is squelched. However, if the anti-apoptotic reserve is overcome by the surge in BH3-only signaling, BAX/BAK activation and self-association proceed, leading to poration of the mitochondrial outer membrane. As a consequence, key apoptogenic factors are released from the mitochondria, activating enzymes that degrade the cell's protein and nucleic acid contents in an orchestrated cellular suicide.
BOX 2. BH3 Domain Interactions: A Tale of Two Sites.
A series of X-ray and NMR structures of apoptotic proteins and their complexes with BH3 domains demonstrate that the key BH3 interaction motif of BCL-2 family proteins forms an amphipathic α-helix, either constitutively or when bound to its target. The BH3 domain of pro-apoptotic proteins is required for killing activity and can be blocked by anti-apoptotic BCL-2 family members. The first structure of a BCL-2 family protein, anti-apoptotic BCL-XL, revealed the presence of a surface hydrophobic groove113 that accommodates the BH3 helix through interaction with its hydrophobic surface and perimeter of charged and hydrophilic residues27. This seminal work defined a key mode of anti-apoptotic protein interaction that blocks both the pro-apoptotic effectors BAX and BAK, and the BH3-only death sentinels, such as BIM and BAD. Indeed, this canonical BH3-binding site of anti-apoptotic proteins is a major pharmacologic target for overcoming the apoptotic blockades of diseases such as cancer.
A structural and biochemical investigation of the protein interaction-based mechanism underlying BH3-medicated direct BAX activation uncovered a second BH3 interaction site on pro-apoptotic BAX58. This novel site is likewise defined by a surface hydrophobic groove surrounded by charged and hydrophilic amino acids that engage the amphipathic BIM BH3 α-helix in complementary fashion. In contrast to BH3 binding to the canonical pocket, which is located at the C-terminal side of the protein and produces a stable inhibitory complex, the novel site on BAX is found on the N-terminal face, and when engaged by BIM BH3, a dynamic structural reorganization of the BAX protein ensues68. The identification of this second BH3-binding site broadens the paradigm for BCL2 family interactions and how protein function can be modulated through BH3 engagement at two distinct regulatory sites.
BOX 3. Indirect and Direct Activation of BAX/BAK as a Therapeutic Strategy: Is it Safe?
Nature has installed a sophisticated security system to prevent renegade activation of BAX/BAK due to the attendant threat of unchecked mitochondrial apoptosis to organism homeostasis. Thus, wanton pharmacologic activation of BAX/BAK, whether by indirect or direct activators, would at first blush appear to be risky business. However, the preclinical and early clinical data suggest that the differences in apoptotic reserve among normal and diseased cells might provide a practical therapeutic window. Efficacy studies involving short-term SAHB treatment of mice bearing human cancer xenografts revealed suppression of tumor growth without damage to normal tissues56. The small molecule BCL2/BCL-XL inhibitor ABT-263 has been generally well-tolerated in early clinical trials101, 102, with at least one side effect directly related to on-target biological activity114. Whereas unstressed cells may have a sufficient pool of unoccupied anti-apoptotic proteins to withstand the pharmacologic hit and maintain BH3 suppression, cancer cells – bombarded by external and internal death signals from high metabolic demands, hypoxia, genetic damage, unfolded proteins, and others stressors – may acutely respond to indirect and direct BAX activation due to an overwhelmed anti-apoptotic reserve. Importantly, the distinction between resistant and susceptible cells is not simply based on the quantity of survival proteins expressed, but whether such proteins are unbound and thus capable of withstanding pharmacologic assault, or saturated with the BH3 helices of pro-apoptotic proteins and thus “primed for death”49. For example, two cancers that are “addicted” to BCL-2 and overexpress the protein at similar high levels may respond quite differently to a targeted BCL-2 family therapeutic if one cancer maintains a robust pool of unbound BCL-2, causing resistance, and the other is laden with BCL-2–pro-apoptotic protein complexes, setting the stage for drug sensitivity. Understanding and navigating this therapeutic window will be essential to the success of experimental therapeutics designed to target the BCL-2 family interaction network.
BOX 4. Outstanding Questions.
Do protein interaction mechanisms outside of the BCL-2 family network regulate BAX activation? If so, how?
How does the lipid environment influence BAX interactions and function?
What is the structure of the BAX homo-oligomer?
What are the proteolipid components of the BAX-containing mitochondrial outer membrane pore?
How do the differential conformations of BAX influence its non-apoptotic roles in mitochondrial morphology and calcium homeostasis?
Can pharmacologic targeting of the BAX trigger site or its conformers be exploited to modulate BAX for therapeutic benefit in diseases of deregulated apoptosis?
Acknowledgements
We thank E. Smith for figure design. This work was supported by NIH grant 5R01CA50239 to L.D.W. and 1K99HL095929 to E.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pegoraro L, et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A. 1984;81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakhshi A, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 3.Cleary ML, Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985;82:7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell TJ, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 6.Vaux DL, et al. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 7.Oltvai ZN, et al. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 8.Kozopas KM, et al. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boise LH, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 10.Choi SS, et al. A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow. Oncogene. 1995;11:1693–1698. [PubMed] [Google Scholar]
- 11.Lin EY, et al. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993;151:1979–1988. [PubMed] [Google Scholar]
- 12.Gibson L, et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- 13.Ke N, et al. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J Biol Chem. 2001;276:12481–12484. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- 14.Chittenden T, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 15.Farrow SN, et al. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 16.Kiefer MC, et al. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 17.Hsu SY, et al. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci U S A. 1997;94:12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockenbery D, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 19.Monaghan P, et al. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992;40:1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- 20.Krajewski S, et al. Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res. 1996;56:2849–2855. [PubMed] [Google Scholar]
- 21.Wolter KG, et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 23.Jurgensmeier JM, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 25.Chittenden T, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, et al. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 27.Sattler M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 28.Cosulich SC, et al. Regulation of apoptosis by BH3 domains in a cell-free system. Curr Biol. 1997;7:913–920. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, et al. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang E, et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 31.Boyd JM, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 32.Han J, et al. Induction of apoptosis by human Nbk/Bik, a BH3- containing protein that interacts with E1B 19K. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, et al. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor L, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis SN, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 36.Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 37.Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 38.Wei MC, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarou M, et al. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J Biol Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng EH, et al. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 41.Edlich F, et al. Bcl-x(L) Retrotranslocates Bax from the Mitochondria into the Cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo B, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M, et al. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 44.Lovell JF, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Oh KJ, et al. A membrane-targeted BID BCL-2 homology 3 peptide is sufficient for high potency activation of BAX in vitro. J Biol Chem. 2006;281:36999–37008. doi: 10.1074/jbc.M602341200. [DOI] [PubMed] [Google Scholar]
- 46.Terrones O, et al. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- 47.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Kuwana T, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 49.Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 51.Walensky LD, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Eskes R, et al. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez D, White E. TNF-alpha signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol Cell. 2000;6:53–63. [PubMed] [Google Scholar]
- 54.Chou JJ, et al. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 55.McDonnell JM, et al. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 56.Walensky LD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafmeister CE, et al. An all-hydrocarbon crosslinking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]
- 58.Gavathiotis E, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlesinger PH, et al. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antonsson B, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 61.Hsu YT, et al. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 63.Khaled AR, et al. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cartron PF, et al. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 2004;578:41–46. doi: 10.1016/j.febslet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 65.Pagliari LJ, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci U S A. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desagher S, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 68.Gavathiotis E, et al. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Upton JP, et al. The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death Differ. 2007;14:932–942. doi: 10.1038/sj.cdd.4402092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nechushtan A, et al. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim H, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cartron PF, et al. Involvement of the N-terminus of Bax in its intracellular localization and function. FEBS Lett. 2002;512:95–100. doi: 10.1016/s0014-5793(02)02227-5. [DOI] [PubMed] [Google Scholar]
- 73.Cartron PF, et al. Distinct domains control the addressing and the insertion of Bax into mitochondria. J Biol Chem. 2005;280:10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 74.Schinzel A, et al. Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George NM, et al. Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process. J Biol Chem. 2010;285:1384–1392. doi: 10.1074/jbc.M109.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meijerink JP, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- 77.Meijerink JP, et al. Bax mutations in cell lines derived from hematological malignancies. Leukemia. 1995;9:1828–1832. [PubMed] [Google Scholar]
- 78.Czabotar PE, et al. Mutation to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–7131. doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan C, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764–14775. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du C, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 82.Antonsson B, et al. Bax oligomerization is required for channel- forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- 83.Antonsson B, et al. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 84.Epand RF, et al. Direct evidence for membrane pore formation by the apoptotic protein Bax. Biochem Biophys Res Commun. 2002;298:744–749. doi: 10.1016/s0006-291x(02)02544-5. [DOI] [PubMed] [Google Scholar]
- 85.Dewson G, et al. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Dewson G, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Ding J, et al. Bcl-2 and Bax interact via the BH1-3 groove-BH3 motif interface and a novel interface involving the BH4 motif. J Biol Chem. 2010;285:28749–28763. doi: 10.1074/jbc.M110.148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia-Saez AJ, et al. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry. 2004;43:10930–10943. doi: 10.1021/bi036044c. [DOI] [PubMed] [Google Scholar]
- 89.Garcia-Saez AJ, et al. Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys J. 2005;88:3976–3990. doi: 10.1529/biophysj.104.058008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Saez AJ, et al. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006;273:971–981. doi: 10.1111/j.1742-4658.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- 91.George NM, et al. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bleicken S, et al. Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christenson E, et al. Cholesterol effects on BAX pore activation. J Mol Biol. 2008;381:1168–1183. doi: 10.1016/j.jmb.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Landeta O, et al. Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process. J Biol Chem. 2011;286:8213–8230. doi: 10.1074/jbc.M110.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang G, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 96.Stewart ML, et al. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen M, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rampino N, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, et al. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 101.Gandhi L, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson WH, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knudson CM, et al. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 104.Deckwerth TL, et al. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 105.Perez GI, et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 106.Dong H, et al. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hochhauser E, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 108.Ben-Ari Z, et al. Bax ablation protects against hepatic ischemia/reperfusion injury in transgenic mice. Liver Transpl. 2007;13:1181–1188. doi: 10.1002/lt.21221. [DOI] [PubMed] [Google Scholar]
- 109.Hochhauser E, et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 110.Yang L, et al. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:648–654. doi: 10.1167/iovs.03-0827. [DOI] [PubMed] [Google Scholar]
- 111.Hetz C, et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- 112.Bombrun A, et al. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J Med Chem. 2003;46:4365–4368. doi: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- 113.Muchmore SW, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 114.Mason KD, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]