Abstract
This report reviews and discusses the existing evidence on recommended FSH doses in IVF cycles. A comprehensive search for relevant data was performed in the Cochrane Library, MEDLINE, EMBASE and NICE clinical guidelines database, and the reference lists of manuscripts. A good body of evidence exists looking at starting doses of FSH for first IVF attempts. Only one randomised controlled trial was identified looking at the efficacy of increasing doses during an IVF cycle. Some observational studies dealt with the question whether a suboptimal ovarian response should be managed by an increased FSH dose in a subsequent cycle, but no randomised trials addressed this issue.
Keywords: IVF, FSH, Dose, Stimulation, Poor responder, Review
Introduction
Ovarian stimulation
The discovery of controlled ovarian hyperstimulation has been regarded as a crucial breakthrough for IVF without which it may never have become a viable infertility treatment [1]. Although natural cycle IVF is still practiced by some centers, the ongoing pregnancy rate is estimated to be only 7.2% with a cancellation rate of almost 30% [2]. In contrast to natural cycle IVF, controlled ovarian hyperstimulation leads to the retrieval of multiple oocytes in one attempt. The careful selection of the best quality embryo(s) is a critical step in achieving the high clinical pregnancy rates most centers now enjoy. In addition, the potential cryopreservation of supernumerary embryos can further add to the cumulative pregnancy rate achieved with one egg retrieval.
Several strategies exist to bring about the recruitment and growth of multiple follicles. By far, the most widely used techniques are based on a combination of suppression of the pituitary-gonadal axis to prevent ovulation and direct ovarian stimulation.
Ovarian stimulation for IVF is generally performed with follicle stimulating hormone (FSH). Both urinary-derived and recombinant FSH preparations are available. Despite purification, urinary-derived FSH preparations contain low levels of contaminants, including LH. The co-administration of low levels of LH can be beneficial in conditions where trace amounts are absent, such as in women with Kallman syndrome. A review funded by the National Institute of Clinical Excellence (NICE), however, showed no difference in clinical effectiveness between the two types of preparations (Evidence level 1a) for women with normal LH levels [3]. In Australia, however, only recombinant human FSH (recFSH) is approved for the induction of multiple follicular growth [4].
Given its acknowledged importance in the success of IVF one would expect there to be clear guidelines regarding the appropriate dose of FSH for different clinical situations. However, no such consensus seems to exist and a variety of dosing regimens are advocated. Whether the dose should be adjusted to the initial response or kept constant throughout stimulation is a similar source of contention.
Poor responders
An even more difficult problem is the treatment of the ‘poor responder.’ From a practical point of view a distinction can be made between those women we expect to respond poorly and those who have already had—not necessarily expected—a poor response in a previous cycle.
Women expected to have a poor response might be those of older age, those with fewer antral follicles, those with high basal FSH concentrations or low basal inhibin B and/or AMH concentrations. These measures and others are all soft markers for an individual’s ovarian response to stimulation. None of these markers, either separately or in combination, are predictive enough to justify refusal of treatment in non-menopausal patients [5].
Women with a previous poor response to ovarian stimulation make up the other group. There appears to be no consensus about the definition of a poor response in the literature, however. As Surrey and Schoolcraft [6] point out in their review of the topic, this lack of agreement on who constitutes a poor responder also makes it very difficult to critically evaluate the different treatment strategies for this challenging patient subset (Table 1).
Table 1.
Summary of criteria used in studies on “poor responders”
| Criteria |
|---|
| No. of mature follicles |
| FSH levels in early follicular phase |
| “Efficacy index” |
| Maximum estradiol levels |
| Age > 40 years |
| Mean daily gonadotropin dose |
| Total gonadotropin dose |
| Days of gonadotropin treatment |
| Number of mature oocytes retrieved |
| Single dominant follicle |
| Spontaneous LH surge |
| Failed “Lupron screening test” |
| Failed conception |
| Undefined |
Adapted from: Surrey et al. Fertil Steril 2000 [6].
Aims
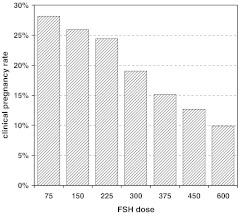
This review will examine what starting doses of FSH may be appropriate in the first and subsequent IVF attempts. Dosing strategies for the ‘poor responder’ will be reviewed in more detail. This patient population presents a specific problem because there appears to be little consensus on the maximum effective dose. This is not a mere pharmacological question, as it also raises concerns from a health economic point of view, illustrated by the inverse relationship between FSH starting dose and clinical pregnancy rate (Fig. 1).
Fig. 1.
Clinical pregnancy rates at Monash IVF grouped per starting dose for nafarelin down-regulated cycles (with or without pill-scheduling) between 1997–2006
Methods
A comprehensive search for data was performed in the Cochrane Library, MEDLINE, EMBASE and NICE clinical guidelines database up until July 2006. Search terms included FSH, dose, stimulation, poor responder, randomized and RCT. Reference lists of relevant manuscripts were hand searched. All studies, retrospective and prospective, addressing dosing strategies as outlined in the aims were reviewed. The main outcome measure of interest was the clinical pregnancy rate per cycle started. Secondary outcome measures were the number of oocytes, high quality embryos and frozen embryos. The debate of whether the addition of LH improves the ovarian response is a separate one. Thus, preference was given to studies investigating the effectiveness of recFSH where sufficient data was available.
A subgroup of five RCTs with very similar designs comparing 100 vs. 200 IU recFSH in normal responders was identified. For this subgroup the clinical pregnancy rates per cycle started were expressed as an odds ratio, with 95% confidence intervals using a random effects model.
Starting dose for the first IVF attempt
Expected normal responders
Several clinical predictors are reported to assist in determining which patients might be normal responders [7]. These factors include, amongst others, female age, early follicular phase serum concentrations of FSH, inhibin B and anti-Müllerian hormone and ultrasound findings. A reasonable assessment of a patient’s expected response can often be made [8] despite the fact that none of the currently available tests are predictive enough to provide clear diagnostic cut-offs [5].
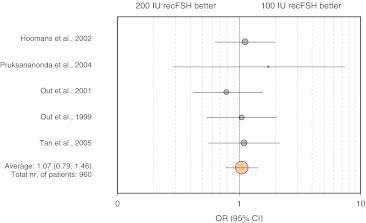
A significant number of randomised controlled trials (RCTs) were identified that address the question of the FSH starting dose in expected normal responders. Using gonadotrophin-releasing hormone (GnRH) agonists for pituitary downregulation, five studies [9–13] compared a starting dose of 100 IU with 200 IU and three studies [14–16] compared a starting dose of 150 IU with 250 IU. Two studies were identified that used GnRH-antagonists for pituitary suppression. One compared a starting dose of 150 IU with 200 IU [17], whereas the other compared 150 IU with 225 IU [18]. A summary of the relevant data is provided in Table 2 and Fig. 2. All the studies were designed to detect differences in the number of oocytes collected and the total dose of FSH used and were of similar quality. Although the studies were not powered to detect small, but possibly clinically relevant differences in pregnancy rates, they did report them as secondary outcomes. Figure 2 summarizes the results of a meta-analysis for the five studies comparing 100 IU with 200 IU. It reveals little prospect for larger trials to detect a statistically significantly higher pregnancy rate in favor of the higher dose.
Table 2.
RCTs comparing different starting doses in expected normal responders
| Patient numbers | Age group | Oocytes collected | CPR(%)/Start | |
|---|---|---|---|---|
| GnRH Agonist | ||||
| 100 vs. 200 IU | ||||
| Tan et al. [9] | 192 | 18–39 | 10.9 vs. 12.2 (NS) | 27 vs. 24 (NS) |
| Out et al. [10] | 199 | 18–39 | 6.2 vs. 10.6 (P < 0.001) | 24 vs. 23 (NS) |
| Out et al. [11] | 179 | 18–37 | 5.7 vs. 12.0 (P < 0.001) | 20 vs. 25 (NS) |
| Pruksananonda et al. [12] | 60 | 25–38 | 6.0 vs 9.2 (NS) | 23 vs. 13 (NS) |
| Hoomans et al. [13] | 330 | 18–39 | 5.0 vs. 9.6 (P < 0.001) | 20 vs. 18 (NS) |
| 150 vs. 250 IU | ||||
| Out et al. [14] | 138 | 30–39 | 9.1 vs. 10.6 (NS) | 24 vs. 18 (NS) |
| Yong et al. [15] | 124 | 23–41 | 6.3 vs. 8.3 (P < 0.05) | 15 vs. 19 (NS) |
| Latin-American Puregon IVF Study Group. 2001 [16] | 404 | 30–39 | 8.9 vs. 10.2 (NS) | 23 vs. 24 (NS) |
| GnRH Antagonist | ||||
| 150 vs. 200 IU | ||||
| Out et al. [17] | 257 | 18–39 | 10.3 vs. 11.9 (NS) | 31 vs. 25 (NS) |
| 150 vs. 225 IU | ||||
| Wikland et al. [18] | 120 | 20–39 | 9.1 vs. 11.0 (P < 0.023) | 26 vs. 25 (NS) |
Fig. 2.
Odds ratio (OR) for clinical pregnancy rates for RCTs comparing 100 vs. 200 IU recFSH. Circles represent point estimates of OR. Circle size is proportionate to number of patients. The 95%CI are illustrated by error bars. The overall OR was calculated using the Peto method. There was no statistically significant heterogeneity between the studies (χ2 = 1.97; P = 0.74)
Taken together these RCTs suggest that higher starting doses do not lead to improved pregnancy rates, despite lower cancellation rates. One could argue that the higher oocyte numbers associated with higher starting doses will lead to more frozen embryos and therefore better cumulative pregnancy rates. However, seven of the ten RCTs found no significant difference. A higher number of frozen embryos was only found in three of the five studies comparing 100 vs. 200 IU. Although it remains to be proven, it perhaps suggests that a starting dose of 100 IU may be associated with lower cumulative pregnancy rates per oocyte collection.
Even so, the improved egg yield was only apparent in younger patients, arguably those that need it the least. In a study by Out et al. [14] in the patient group of 30–33 years an average of four more eggs was collected (15 vs. 11 eggs) if patients were given 250 IU instead of 150 IU. In the two other groups (34–36 years and 37–39 years) there was no difference. This observation was echoed by findings from the study from Yong et al. [15]. They reported that approximately five more eggs were retrieved in women aged <33 years in the 225-IU compared with the 150-IU group, but in older women (≥33 years), the number of eggs retrieved in both groups was similar.
In view of the above, the higher rate of hormone-related side-effects and extra cost associated with the higher dose protocol, it would be reasonable to conclude that most patients should start on a dose of 150 IU.
Expected poor responders
There is only one randomised controlled trial that looked at the efficacy of doubling the starting dose in women who are anticipated to respond poorly [19]. Those patients with <5 antral follicles just prior to starting gonadotrophins were recruited for the study. Fifty-two patients were randomised to the normal starting dose of 150 IU/day or the higher dose of 300 IU/day. The median number of oocytes and embryos was the same for both groups (three and two, respectively).
Increasing the dose during the course of an IVF cycle
When cycle monitoring indicates that the ovarian response has been unsatisfactory, some centers advocate adjusting the dose to rescue the cycle. How effective such dose increases are, is not well established.
Despite this, the practice still appears well accepted. For example, two major RCTs [20, 21] reported at the 2006 Annual Meeting of European Society of Human Reproduction and Embryology allowed for the recFSH dose to be adjusted, after a 6–7 days course of a fixed dose. This belief that the ovary may respond better after increasing the dose does not seem to be borne out by the evidence.
This review identified one prospective randomized study and two retrospective studies. The RCT clearly demonstrated that doubling the human menopausal gonadotrophin (hMG) dose in patients with a low response after 5 days of 225 IU hMG, did not result in a higher number of oocytes retrieved compared to patients treated with a fixed dose [22]. The two retrospective studies also concluded that a low response after 5 days of hMG therapy may not be averted by doubling or increasing the dose of hMG [23, 24].
Dose following a poor response
There are probably as many suggested treatment strategies as there are definitions for patients who have had a previous poor response [25]. An often-cited strategy is to increase the dose of gonadotrophins in the next cycle. Instinctively, this may appear unavoidable when faced with the lack of any initial ovarian responsive to a lower dose, but the lack of good quality supportive evidence is remarkable. Indeed, in the NICE guidelines this common problem is reduced to the following line: “There is minimal or no benefit with the use of increased dose of gonadotrophins” [26].
Although no RCTs were found that directly addressed the issue, the answer can perhaps be deduced from other RCTs. As mentioned previously, the RCTs by Out et al. [14] and Yong et al. [15] elegantly demonstrated that higher starting doses of FSH in normal responders do not result in more eggs once they are over the age of 33 years. Similarly, expected poor responders experienced no benefit from being started on a dose of 300 IU instead of 150 IU [19]. If they do not already do so in the first cycle, it seems irrational to expect older women to respond better to a higher dose in a subsequent cycle.
There is also some evidence from observational studies. Klinkert et al. did an interesting study showing that 64% of the unexpected poor responders and 31% of the expected poor responders had a normal response in the second cycle, most of them after receiving a higher dose of gonadotrophins [27]. According to the authors the difference between both groups suggests that dose increases alone do not always explain a better response in the subsequent cycle. Natural cycle-to-cycle variation, which are more likely in unexpected poor responders, may also account for some of the observed “improvement.”
Three non-randomised studies showed that increasing the dose of hMG up to 450 IU/day in a second cycle did not increase the number of available embryos, nor did it improve the outcome of the treatment cycle compared to the previous cycle in which the patients had been started on a lower hMG dose [28–30]. A retrospective analysis by Land et al. [30] analysed outcomes of 126 patients who had undergone two IVF cycles, the first cycle starting on 225 IU/day and the second cycle starting on 450 IU/day. More oocytes were collected on the higher dose, but the total number of available embryos did not differ.
A similar study by Lashen et al. [31] 2 years later looked at 244 patients who had had at least two cycles of IVF treatment with a higher dose in the second treatment cycle. Although more oocytes were collected in the second cycle, a more detailed analysis revealed that this was only true for those patients who had received ≤150 IU/day in their first cycle. Those who were given 225 or 300 IU/day in the first cycle did not perform any better in their subsequent cycle despite a dose increase.
At best, more eggs appear to be retrieved in a subsequent cycle on a higher dose and it would be tempting to conclude that this is in the patient’s best interest. It should, however, come as no surprise that these non-randomised studies are prone to erroneous conclusions. Firstly, Land’s study failed to show an increase in the number of embryos. Secondly, a lot of the observed improvements can be readily explained by a phenomenon called “regression to the mean,” a term first coined by Galton [32].
The concept of regression to the mean can be illustrated with data from the Monash IVF database. Clinical outcomes from all long down-regulation cycles (with or without pill-scheduling) using nafarelin and 225 IU of recFSH were retrieved. The average number of oocytes retrieved for the whole data set was 11.5.
The first subset of data (Group A) included women who had received 225 IU of recFSH in the first cycle and 300 IU in the second cycle. It is reasonable to assume that the dose was increased because the patient was perceived to have had a sub-optimal response (few oocytes collected). The first column of Table 3 shows that significantly more oocytes were collected in the second cycle on higher dose.
Table 3.
The “regression to the mean” effect
| Group A (n = 135) | Group B (n = 37) | Group C (n = 42) | |
|---|---|---|---|
| 1st cycle | 7.3 ± 3.6 | 4.5 ± 3.7 | 20.2 ± 4.6 |
| 2nd cycle | 9.2 ± 4.9 | 8.7 ± 5.8 | 15.6 ± 6.2 |
| P = 0.00003 | P = 0.0005 | P = 0.0002 |
Numbers represent mean of oocytes collected ±SD.
Group A: women who had received 225 IU of recFSH in the first cycle and 300 IU in the second cycle.
Group B: women who had <10 oocytes collected when receiving 225 IU of recFSH in the first cycle and remained on 225 IU in the second cycle.
Group C: women who had >15 oocytes collected when receiving 225 IU of recFSH in the first cycle and remained on 225 IU in the second cycle.
Pairwise comparison between 1st and 2nd cycle using Paired Student’s t Test.
The second subset (Group B) included women who (a) had received 225 IU of recFSH in the first cycle and (b) had fewer than ten oocytes collected but (c) had remained on 225 IU in the second cycle. Remarkably, there was again a statistically significant increase in the number of oocytes collected in the second cycle despite the fact that no dose increase was ordered.
The third subset (Group C) included women who (a) had received 225 IU of recFSH in the first cycle and (b) had more than 15 oocytes collected and (c) had remained on 225 IU in the second cycle. This time a significant drop in the number of oocytes collected was observed in the second cycle.
The outcomes for Groups B and C clearly illustrate that if a subset of data is selected for outcomes that deviate significantly from the overall mean, it is inevitable that the outcomes in a subsequent cycle will move back or “regress” towards the mean by chance alone. So, while IVF specialists had the best intentions when they increased the dose for women with a low number of oocytes in the first cycle, the observed “improved” outcome can be explained by chance alone. As always, definite proof of treatment effect can only be obtained from a properly designed RCT.
Review of current practice
Tarlatzis et al. have systematically reviewed studies offering treatment alternatives for poor responders and provide an insight in what is considered standard practice [33]. Tarlatzis et al. state that, according to most authors, the most widely used initial dose for poor responders is at least 300 IU/day. A further analysis of the studies in their review reveals that the maximum dose recommended by most authors was 300–450 IU/day. The maximum dose was <300 IU/day in two studies, 300 IU/day in 12 studies, 450 IU/day in 13 studies and 600 IU/day in five studies.
Summary
Lashen et al. concluded their study with two recommendations. Firstly, they suggested that “a national or international analysis of data from many centers using recombinant gonadotropin in IVF might establish a maximum effective dose for this preparation (probably 300 IU/day).” and secondly they argued that “a starting dose of 150 IU per day in the first IVF cycle represents the best compromise between safety and efficacy for the younger IVF patient with a good prognosis for conception within one or three cycles of IVF treatment.”
From the available evidence presented it would seem reasonable to draw the following conclusions:
| Normal Responders |
| Higher starting doses in normal responders lead to more oocytes, butonly in young patients. |
| Clinical pregnancy rates do not improve when higher doses are administered (100 vs. 200 IU or 150 vs. 250 IU). |
| The majority of RCTs fail to show that the retrieval of more oocytes translates in the availability of more frozen embryos. |
| In their first cycle most patients should respond well to 150–200 IU/day. Whether special allowances need to be made for BMI, PCOS, age or other factors is unclear. |
| Poor Responders |
| Increasing the dose of FSH during a cycle is not effective in averting a poor response. |
| There is insufficient evidence for an increased FSH dose after a previous poor response. |
| Although not supported by good evidence most authors seem to be comfortable with a starting dose of 300 IU/day. Similarly, a maximum dose of 450 IU/day seems to be universally accepted. |
References
- 1.Trounson AO, Leeton JF, Wood C, Webb J, Wood J. Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science. 1981;212:681–682. doi: 10.1126/science.7221557. [DOI] [PubMed] [Google Scholar]
- 2.Pelinck MJ, Hoek A, Simons AH, Heineman MJ. Efficacy of natural cycle IVF: a review of the literature. Hum Reprod Updat. 2002;8:129–139. doi: 10.1093/humupd/8.2.129. [DOI] [PubMed] [Google Scholar]
- 3.Fertility: assessment and treatment for people with fertility problems. Commissioned by the National Institute for Clinical Excellence. London: RCOG Press; 2004. pp. 60–61. [PubMed] [Google Scholar]
- 4.Norman RJ, Lacey S, Wang JX. Recombinant or urinary gonadotrophins—an Australian perspective. Reprod Biomed Online. 2005;11:542–543. doi: 10.1016/S1472-6483(10)61157-1. [DOI] [PubMed] [Google Scholar]
- 5.Scott RT., Jr Diminished ovarian reserve and access to care. Fertil Steril. 2004;81:1489–1492. doi: 10.1016/j.fertnstert.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Surrey ES, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fertil Steril. 2000;73:667–676. doi: 10.1016/S0015-0282(99)00630-5. [DOI] [PubMed] [Google Scholar]
- 7.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 8.Popovic-Todorovic B, Loft A, Bredkjæer HE, Bangsboll S, Nielsen IK, Andersen AN. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard’ dose of 150 IU/day in ‘standard’ patients undergoing IVF/ICSI treatment. Hum Reprod. 2003;18:2275–2282. doi: 10.1093/humrep/deg472. [DOI] [PubMed] [Google Scholar]
- 9.Tan SL, Child TJ, Cheung AP, Fluker MR, Yuzpe A, Casper R, et al. A randomized, double-blind, multicenter study comparing a starting dose of 100 IU or 200 IU of recombinant follicle stimulating hormone (Puregon®) in women undergoing controlled ovarian hyperstimulation for IVF treatment. J Assist Reprod Genet. 2005;22:81–88. doi: 10.1007/s10815-005-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Out HJ, Lindenberg S, Mikkelsen AL, Eldar-Geva T, Healy DL, Leader A, et al. A prospective, randomized, double-blind clinical trial to study the efficacy and efficiency of a fixed dose of recombinant follicle stimulating hormone (Puregon®) in women undergoing ovarian stimulation. Hum Reprod. 1999;14:622–627. doi: 10.1093/humrep/14.3.622. [DOI] [PubMed] [Google Scholar]
- 11.Out HJ, David I, Ron-El R, Friedler S, Shalev E, Geslevich J, et al. A randomized, double-blind clinical trial using fixed daily doses of 100 or 200 IU of recombinant FSH in ICSI cycles. Hum Reprod. 2001;16:1104–1149. doi: 10.1093/humrep/16.6.1104. [DOI] [PubMed] [Google Scholar]
- 12.Pruksananonda K, Suwajanakorn S, Sereepapong W, Virutamasen P. Comparison of two different fixed doses of follitropin-beta in controlled ovarian hyperstimulation: a prospective randomized, double blind clinical trial. J Med Assoc Thail. 2004;87:1151–1155. [PubMed] [Google Scholar]
- 13.Hoomans EH, Mulder BB, Asian Purgeon Study Group A group-comparative, randomized, double-blind comparison of the efficacy and efficiency of two fixed daily dose regimens (100- and 200-IU) of recombinant follicle stimulating hormone (rFSH, Puregon) in Asian women undergoing ovarian stimulation for IVF/ICSI. J Assist Reprod Genet. 2002;19:470–476. doi: 10.1023/A:1020358419073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Out HJ, Braat DD, Lintsen BM, Gurgan T, Bukulmez O, Gokmen O, et al. Increasing the daily dose of recombinant follicle stimulating hormone (Puregon) does not compensate for the age-related decline in retrievable oocytes after ovarian stimulation. Hum Reprod. 2000;15:29–35. doi: 10.1093/humrep/15.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Yong PY, Brett S, Baird DT, Thong KJ. A prospective randomized clinical trial comparing 150 IU and 225 IU of recombinant follicle-stimulating hormone (Gonal-F*) in a fixed-dose regimen for controlled ovarian stimulation in in vitro fertilization treatment. Fertil Steril. 2003;79:308–315. doi: 10.1016/S0015-0282(02)04583-1. [DOI] [PubMed] [Google Scholar]
- 16.Latin-American Puregon IVF Study Group A double-blind clinical trial comparing a fixed daily dose of 150 and 250 IU of recombinant follicle-stimulating hormone in women undergoing in vitro fertilization. Fertil Steril. 2001;76:950–956. doi: 10.1016/S0015-0282(01)02844-8. [DOI] [PubMed] [Google Scholar]
- 17.Out HJ, Rutherford A, Fleming R, Tay CC, Trew G, Ledger W, et al. A randomized, double-blind, multicentre clinical trial comparing starting doses of 150 and 200 IU of recombinant FSH in women treated with the GnRH antagonist ganirelix for assisted reproduction. Hum Reprod. 2004;19:90–95. doi: 10.1093/humrep/deh044. [DOI] [PubMed] [Google Scholar]
- 18.Wikland M, Bergh C, Borg K, Hillensjo T, Howles CM, Knutsson A, et al. A prospective, randomized comparison of two starting doses of recombinant FSH in combination with cetrorelix in women undergoing ovarian stimulation for IVF/ICSI. Hum Reprod. 2001;16:1676–1681. doi: 10.1093/humrep/16.8.1676. [DOI] [PubMed] [Google Scholar]
- 19.Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, Velde ER. Expected poor responders on the basis of an antral follicle count do not benefit from a higher starting dose of gonadotrophins in IVF treatment: a randomized controlled trial. Hum Reprod. 2005;20:611–615. doi: 10.1093/humrep/deh663. [DOI] [PubMed] [Google Scholar]
- 20.Nyboe Andersen A, Humaidan P, Fried G, The Nordic rLH Study Group Addition of rLH (Luveris) to rFSH during the final days of follicular maturation in IVF/ICSI treated patients. A Nordic randomized multicenter trial. Hum Reprod. 2006;21(Suppl. 1):O-138. [Google Scholar]
- 21.Fleming R, on behalf of the “Luveris Pre-treatment Group” Pre-treatment with rhLH: respective effects on antral follicular count and ovarian response to rhFSH. Hum Reprod. 2006;21(Suppl. 1):O-139. [Google Scholar]
- 22.Van Hooff MH, Alberda AT, Huisman GJ, Zeilmaker GH, Leerentveld RA. Doubling the human menopausal gonadotrophin dose in the course of an in-vitro fertilization treatment cycle in low responders: a randomized study. Hum Reprod. 1993;8:369–373. doi: 10.1093/oxfordjournals.humrep.a138053. [DOI] [PubMed] [Google Scholar]
- 23.Hock DL, Louie H, Shelden RM, Ananth CV, Kemmann E. The need to step up the gonadotropin dosage in the stimulation phase of IVF treatment predicts a poor outcome. J Assist Reprod Genet. 1998;15:427–430. doi: 10.1007/BF02744936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalaf Y, Tooukhy T, Taylor A, Braude P. Increasing the gonadotrophin dose in the first course of an in vitro fertilization cycle does not rectify an initial poor response. Eur J Obstet Gynaecol Reprod Biol. 2002;103:146–149. doi: 10.1016/S0301-2115(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 25.Loutradis D, Drakakis P, Milingos S, Stefanidis K, Michalas S. Alternative approaches in the management of poor response in controlled ovarian hyperstimulation (COH) Ann N Y Acad Sci. 2003;997:112–119. doi: 10.1196/annals.1290.013. [DOI] [PubMed] [Google Scholar]
- 26.Fertility: assessment and treatment for people with fertility problems. Commissioned by the National Institute for Clinical Excellence. London: RCOG Press; 2004. p. 102. [PubMed] [Google Scholar]
- 27.Klinkert ER, Broekmans FJ, Looman CW, Velde ER. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil Steril. 2004;81:1247–1253. doi: 10.1016/j.fertnstert.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Karande VC, Jones GS, Beeck LL, Muasher SJ. High dose FSH stimulation at the onset of the menstrual cycle does not improve the in vitro fertilization outcome in low responder patients. Fertil Steril. 1990;53:486–489. doi: 10.1016/s0015-0282(16)53345-7. [DOI] [PubMed] [Google Scholar]
- 29.Pantos C, Thornton SJ, Speirs AL, Johnston I. Increasing the human menopausal gonadotropin dose—does the response really improve? Fertil Steril. 1990;53:436–439. doi: 10.1016/s0015-0282(16)53337-8. [DOI] [PubMed] [Google Scholar]
- 30.Land JA, Yarmolinskaya MI, Dumoulin JCM, Evers JLH. High-dose human menopausal gonadotrophin stimulation in poor responders does not improve in-vitro fertilization outcome. Fertil Steril. 1996;65:961–965. doi: 10.1016/s0015-0282(16)58269-7. [DOI] [PubMed] [Google Scholar]
- 31.Lashen H, Ledger W, Bernal AL, Evans B, Barlow D. Superovulation with a high dose for in vitro fertilization: is it effective? J Assist Reprod Genet. 1998;15:438–443. doi: 10.1007/BF02744938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galton F. Regression towards mediocrity in hereditary stature. J Anthropol Inst. 1886;15:246–263. [Google Scholar]
- 33.Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Updat. 2003;9:61–76. doi: 10.1093/humupd/dmg007. [DOI] [PubMed] [Google Scholar]