Abstract
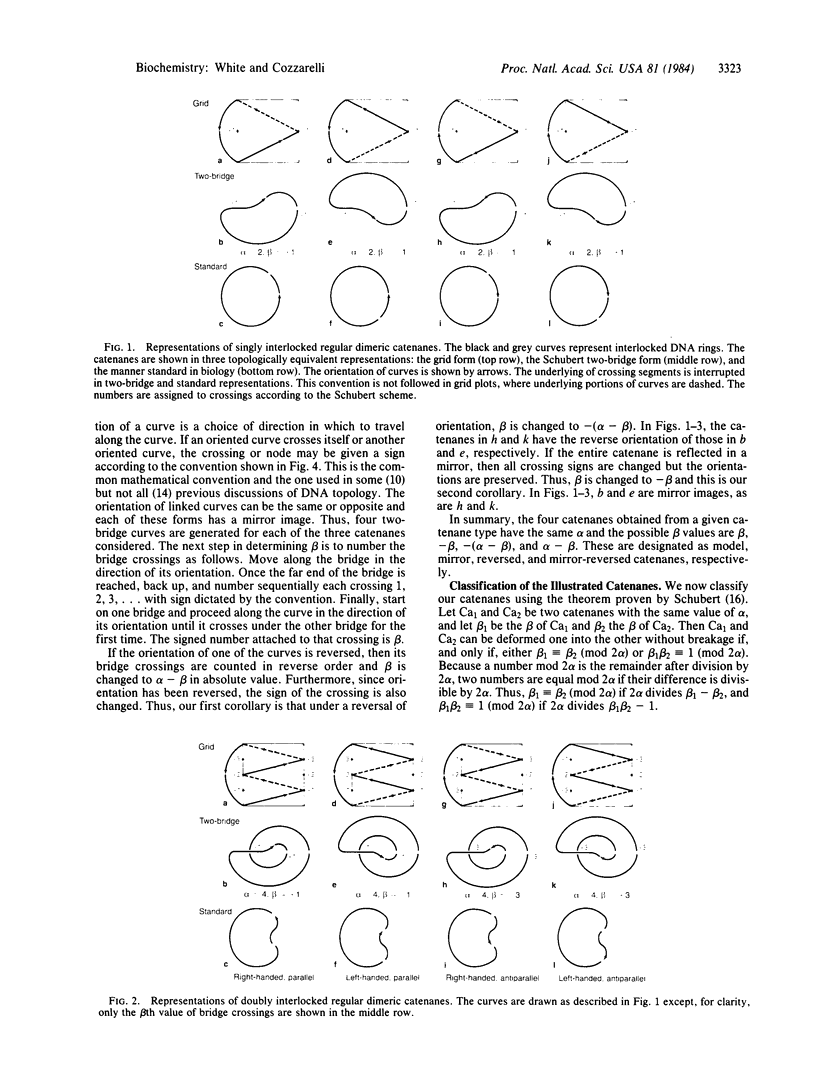
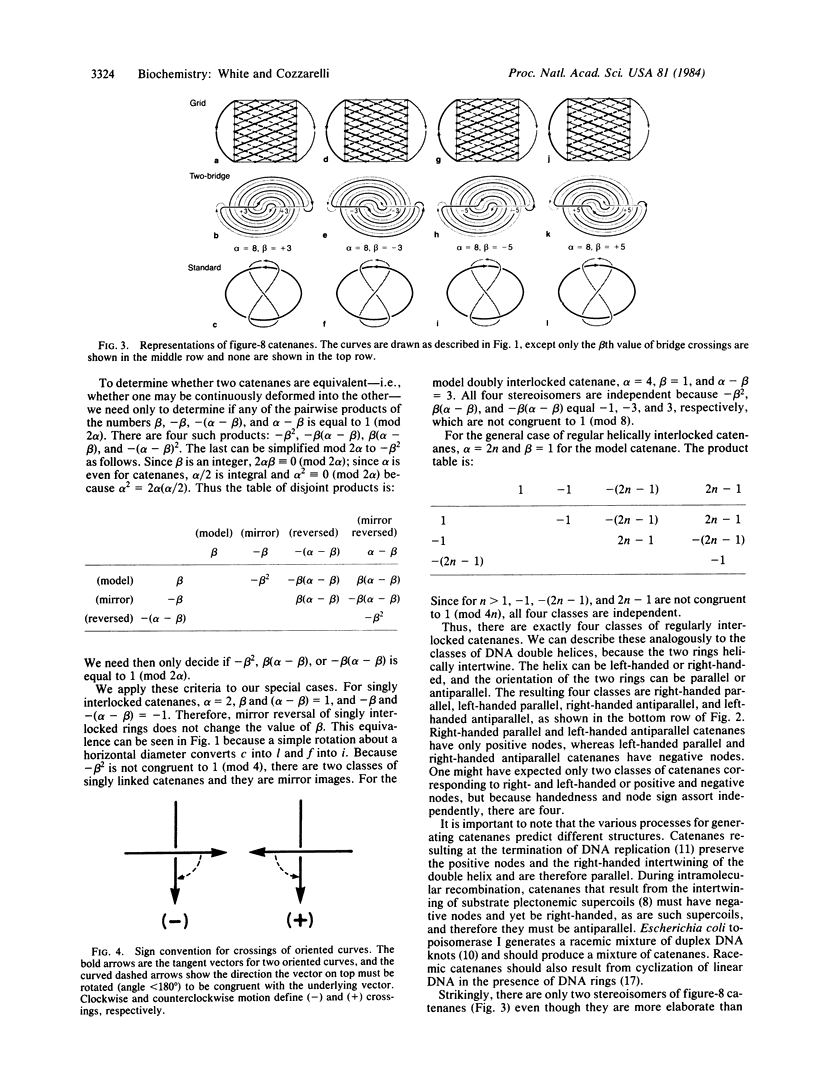
Although linking number is an effective topological invariant for describing supercoiled DNA, it is inadequate for the additional interwinding in catenated or knotted DNA. We explain how the two-bridge theory of Schubert provides a powerful yet simple method for analyzing these forms by associating them with two integral invariants, alpha and beta, that measure their geometric complexity. These integers can either be determined graphically or computed with the aid of standard tables, and they allow tabulation of all possible stereoisomers of a given knot or catenane . A complete classification can then be made via a simple theorem. Stereoisomers of representative knots and catenanes are tabulated for easy reference. There are four stereoisomers of regularly interlocked catenanes that we designate right-handed parallel, right-handed antiparallel, left-handed parallel, and left-handed antiparallel according to the helical intertwining of the rings. The biological processes that form catenanes --replication, recombination, and topoisomerase action--predict distinctly different isomers.
Full text
PDF

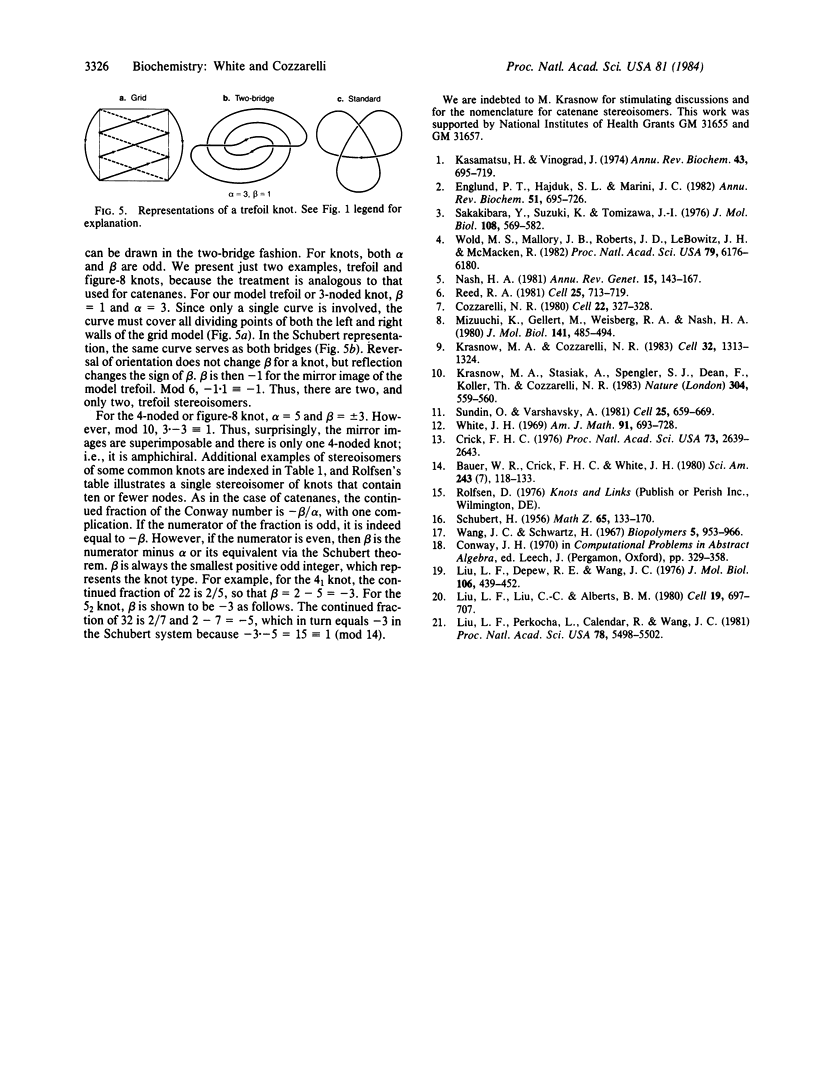
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozzarelli N. R. DNA topoisomerases. Cell. 1980 Nov;22(2 Pt 2):327–328. doi: 10.1016/0092-8674(80)90341-4. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Linking numbers and nucleosomes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2639–2643. doi: 10.1073/pnas.73.8.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Site-specific relaxation and recombination by the Tn3 resolvase: recognition of the DNA path between oriented res sites. Cell. 1983 Apr;32(4):1313–1324. doi: 10.1016/0092-8674(83)90312-4. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Stasiak A., Spengler S. J., Dean F., Koller T., Cozzarelli N. R. Determination of the absolute handedness of knots and catenanes of DNA. Nature. 1983 Aug 11;304(5926):559–560. doi: 10.1038/304559a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Depew R. E., Wang J. C. Knotted single-stranded DNA rings: a novel topological isomer of circular single-stranded DNA formed by treatment with Escherichia coli omega protein. J Mol Biol. 1976 Sep 15;106(2):439–452. doi: 10.1016/0022-2836(76)90095-4. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Perkocha L., Calendar R., Wang J. C. Knotted DNA from bacteriophage capsids. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5498–5502. doi: 10.1073/pnas.78.9.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Weisberg R. A., Nash H. A. Catenation and supercoiling in the products of bacteriophage lambda integrative recombination in vitro. J Mol Biol. 1980 Aug 25;141(4):485–494. doi: 10.1016/0022-2836(80)90256-9. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Parker D. E. The vestibular apparatus. Sci Am. 1980 Nov;243(5):118–135. doi: 10.1038/scientificamerican1180-118. [DOI] [PubMed] [Google Scholar]
- Reed R. R. Transposon-mediated site-specific recombination: a defined in vitro system. Cell. 1981 Sep;25(3):713–719. doi: 10.1016/0092-8674(81)90178-1. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Suzuki K., Tomizawa J. I. Formation of catenated molecules by replication of colicin E1 plasmid DNA in cell extracts. J Mol Biol. 1976 Dec 15;108(3):569–582. doi: 10.1016/s0022-2836(76)80137-4. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Schwartz H. Noncomplementarity in base sequences between the cohesive ends of coliphages 186 and lambda and the formation of interlocked rings between the two DNA's. Biopolymers. 1967;5(10):953–966. doi: 10.1002/bip.1967.360051008. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Mallory J. B., Roberts J. D., LeBowitz J. H., McMacken R. Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6176–6180. doi: 10.1073/pnas.79.20.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]