Abstract
Purpose: The pregnancy outcome and the chances of birth were assessed according to embryo quality after IVF or ICSI.
Methods: The implantation rate (IR), the loss of gestational sacs rate (LGSR), and birth rate (BR) were determined according to the cleavage stage and the integrity of blastomeres after day-2 homogeneous embryo transfers (n = 1812).
Results: The LGSR was higher after transfers of 2–3-cell or 5–6-cell embryos and was significantly increased when more than 20% of the embryo volume was fragmented in 4-cell embryos. After transfers of 4-cell embryos without fragmentation, the BR was significantly higher than the BR after transfers of 4-cell embryos with 1–20% fragmentation (16.6% vs 13.1%). The difference was the consequence of a higher IR (20.4% vs 17.3%) but also of a lower LGSR (18.9% vs 24.2%).
Conclusions: Not only implantation and the ability to give a pregnancy, but also the capacity to give a live birth are dependent on the embryo quality.
Keywords: Assisted reproduction, Embryo development, Embryo quality, IVF/ICSI outcome
Introduction
Embryo quality has been suggested to be of paramount importance to predict the occurrence of pregnancy after In Vitro Fertilization (IVF) [1, 2] but only one study reported the impact of embryo fragmentation on pregnancy and perinatal outcomes [3]. Various morphological factors have been proposed to identify embryos with the best chances of implantation. Embryo fragmentation and cleavage stage have been described as directly influencing the outcome of IVF [4–6]. However, as most studies analyzed transfers of embryos with heterogeneous fragmentation and/or cleavage stages, it was difficult to determine which of the embryos had been implanted and thus the respective importance of these two indicators.
The characterisation of embryos with the best chance of implantation was substantially improved by the analysis of single embryo transfers or “homogeneous” transfers (HT), where homogenous transfer is defined as the transfer of embryos with a similar morphology. Overall, the best implantation rate was observed after transfer of embryos with 4 cells and less than 10% fragmentation at day 2 [7–18], but the pregnancy outcome was not analysed according to the embryo morphology in these studies.
To determine if the morphological aspect of an early embryo affects its capacity to lead to a live birth, data of embryo homogeneous transfers 2 days after IVF and ICSI were analysed.
Materials and methods
All homogeneous transfers of unfrozen embryos (HT), defined as the transfer of embryos with the same number of blastomeres and the same proportion of fragmentation as described below performed two days after the oocyte collection between January 1997 and October 2003 in Cochin–Saint-Vincent de Paul Hospital were analyzed in this study.
IVF/ICSI protocol
Multiple follicular growth was induced by various protocols as described elsewhere [19]. The procedures involved desensitization with GnRH analogues and subsequent administration of exogenous gonadotropins. After hCG administration, oocytes were collected transvaginally under ultrasound guidance. Oocytes were fertilized and embryos were developed in 30 μl of medium covered with mineral oil at 37°C under a 5% CO2 humidified gas atmosphere. HTF (Clinisciences, Montrouge, France) supplemented with serum from the patient was used as culture medium for 69.3% of the attempts, Ménézo B2 medium (C.C.D., Paris, France) was used for 22% of the attempts, and Universal IVF (Medicult, Lyon, France) was used in the 8.7% of the remaining attempts. The choice of ICSI or conventional IVF as fertilization method was dependent on semen sample characteristics and couple’s history. Fresh or frozen-thawed partner (n = 1759) or donor (n = 53) sperm were used. The results were similar regardless of the technique of in vitro fertilization or the culture medium used. So, the results of transfers were combined for the following analysis.
Embryo morphology assessment
Oocytes were observed under an inverted microscope equipped with Hoffman Modulation Contrast® optics (Nikon – TE2000-S – Champigny sur Marne – France) for evidence of fertilization 18 h after insemination or microinjection. Oocytes exhibiting 2 pronuclei were considered as normally fertilized. Cleaved embryos from normally fertilized oocytes were independently assessed for blastomeric fragmentation and for cleavage stage 44 ± 2 h after insemination or microinjection (day 2) by at least two observers, using the same inverted microscope at 400X magnification. Blastomeric fragmentation was scored as follows: A = no fragmentation; B = 1–20% by volume of anucleated fragments; C = 21–50% by volume of anucleated fragments; and D = >50% by volume of anucleated fragments. One to three cleaved embryos were transferred 2 days after IVF or ICSI. The number of transferred embryos was depending on the number and quality of embryos available, female age and number of previous treatment cycles.
Data evaluation
Any increase above 20 IU/ml in the βhCG level measured 14 days after embryo transfer identified a biochemical pregnancy. The first ultrasound evaluation was performed 5–6 weeks after the embryo transfer to determine the presence and the number of intrauterine gestational sacs and to diagnose any case of extrauterine pregnancy. Pregnancy was graded as clinical by the observation of at least a gestational sac and the presence of a beating fetal heart at 5–6 weeks.
Four pregnancy outcomes were recorded: miscarriages, ectopic pregnancies, medical abortions and deliveries. The pregnancy rate (PR) was calculated as the ratio between the number of clinical pregnancies and the number of embryo transfers. The early pregnancy loss (EPL) was the ratio between the number of biochemical pregnancies without a gestational sac at the first ultrasound evaluation and the total number of biochemical pregnancies. The implantation rate (IR) was defined as the ratio between the number of gestational sacs detected by the first ultrasound scan and the number of transferred embryos. The late pregnancy loss (LPL) represented the ratio between the sum of miscarriages, ectopic pregnancies and medical abortions and the number of clinical pregnancies. The total pregnancy loss (TPL) was the sum of EPL and LPL. The delivery rate (DR) was defined as the ratio between the number of deliveries and the number of embryo transfers. The birth rate (BR) was calculated as the ratio between the number of children born and the number of transferred embryos. The loss of gestational sacs rate (LGSR) was the ratio between the number of intrauterine gestational sacs that were not followed by the birth of child and the total number of gestational sacs observed.
Statistical analysis
The χ2 test was used for the comparison of binary variables, and continuous variables were compared using the independent Student’s t-test, when appropriate. The significance level was set at 5% (P < 0.05).
Results
During the study, 1812 homogeneous transfers were made after IVF (n = 745) and ICSI (n = 1067) for 1774 couples. The mean female age was 34.5 ± 0.1 years (23–44) in the IVF group and 33.1 ± 0.1 years (18–43) in the ICSI group; the mean male age was 37 ± 0.2 years (23–58) and 36.8 ± 0.2 years (21–62) in the IVF and ICSI groups respectively. A total of 3256 embryos were transferred in 548 single embryos transfers (254 IVF and 294 ICSI) for 532 couples (30%), 1084 dual transfers (399 IVF and 685 ICSI) for 1062 couples (59.9%) and 180 triple transfers (92 IVF and 88 ICSI), for 180 couples (10.1%). The mean number of transferred embryos was 1.9. The transfers resulted in 419 clinical pregnancies (23.1%) and 315 deliveries (17.4%) of 387 children. The implantation rate was 15.5, and 11.9% of transferred embryos went to birth. The overall rate of miscarriage was 21%, and the overall loss of gestational sacs rate (LGSR) was 23.5%.
We found an influence of the embryo quality observed at day-2 on pregnancy outcome. The loss of gestational sac rate was 29.3 and 31.2% when 2–3-cell embryos or 5–6-cell embryos were transferred respectively compared to 22.7% when 4-cell embryos were transferred (Table 1). Similarly, the loss of gestational sac rate was increasing with the fragmentation of transferred embryos. It was 21.5% when embryos without fragmentation were transferred, 24% when embryos with 1–20% fragmentation were transferred and 50% when embryos with more than 20% fragmentation were transferred (Table 1). The significant increase of LGSR with fragmentation was also observed when only 4-cell embryos were transferred.
Table 1.
Loss of Gestational Sacs Rate (LGSR) according to embryo cleavage stage (2–3 cells, 4 cells and 5–6 cells) and blastomeric fragmentation (A = 0%, B= 1–20%, C = 21–50% and D = ≥ 51%)
| Fragmentation | ||||
|---|---|---|---|---|
| A | B | C+D | Total | |
| Cleavage | ||||
| 2–3 cells | 3/8 (37.5) | 8/30 (26.7) | 1/3 (33.3) | 12/41 (29.3) |
| 4 cells | 31/164 (18.9)a, c | 68/281 (24.2)b | 3/4 (75)b, c | 102/449 (22.7) |
| 5–6 cells | 4/5 (80)a | 1/10 (10) | 0/1 | 5/16 (31.2) |
| Total | 38/177 (21.5)d | 77/321 (24) | 4/8 (50)d | 119/506 (23.5) |
LGSR: Number of lost gestational sacs/Total number of gestational sacs.Corrected χ2 test:(a): p < 0.05.(b, c): p < 0.01.
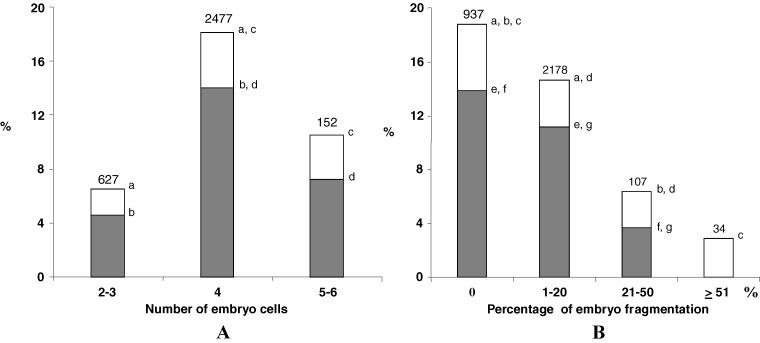
Indeed, the implantation and birth rates were higher when 4-cell embryos were transferred 2 days after fertilization compared to embryos with more or less blastomeres, transferred on the same day (Fig. 1A). The IR and BR also regularly decreased when the embryo fragmentation increased (Fig. 1B). Only 3.7% of transferred embryos went to birth when 21 to 50% of the embryo cell volume was fragmented 2 days after fertilization. When more than 50% of the embryo cell volume was fragmented, no embryo went to birth even if one of them could implant and could induce a pregnancy. We also found a significant decrease of implantation and birth rate when less than 21% of the embryo volume was fragmented compared to embryos without any fragmentation (Fig. 1B).
Fig. 1.
Implantation (white) and birth (grey) rates according to the number of cells (picture A) and the rate of fragmentation (picture B) observed in the embryos, 2 days after IVF or ICSI when they were transferred. Number represents the number of transferred embryos (a, b, c, d, e, f, g : p < 0.05; χ2 test)
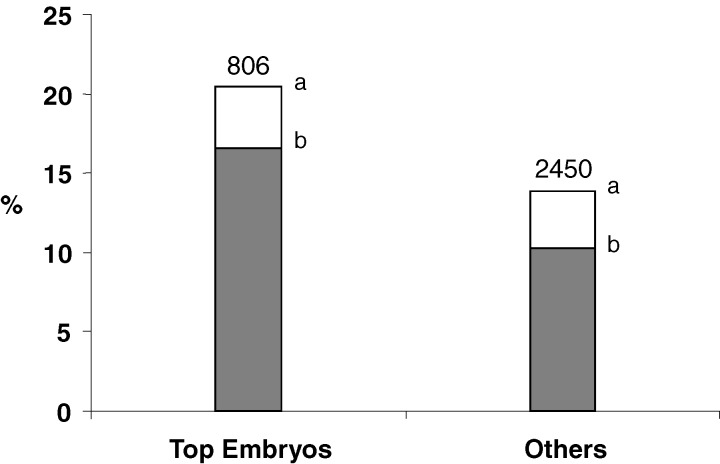
When only 4-cell embryos were transferred 2 days after IVF or ICSI, the birth rate was significantly higher if embryos without any fragmentation (top embryos) were transferred compared to embryos with 1–20% fragmentation (16.5% vs 13.1%; p < 0.05, χ2 test) or compared to all others embryos (16.5% vs 10.4%; p < 0.05, χ2 test–Fig. 2). The better birth rate of 4-cell embryos without fragmentation was the combined consequence of a higher implantation rate (IR: 20.4%) and a lower loss of gestational sac after implantation (LGSR: 18.9%) compared to 4-cell embryos with 1–20% fragmentation (IR: 17.3%, LGSR: 24.2%). This result suggests a better implantation but also a better post implantation development ability of unfragmented 4-cell embryos 2 days after IVF or ICSI.
Fig. 2.
Implantation (white) and birth (grey) rates according to the quality of embryos transferred, 2 days after IVF or ICSI. Top embryos were defined as embryos at 4-cell stage without any fragmentation on the transfer day. Number represents the number of transferred embryos (a,b: p < 0.05; χ2 test)
We confirmed also that the kinetics of the first two cell cycles is a better indicator than the presence of cell fragmentation for subsequent embryo development, since the birth rate of 4-cell embryos with 1–20% fragmentation was significantly higher than the birth rate of embryos without fragmentation but which did not reach the 4-cell stage 2 days after IVF or ICSI (13.1% vs 5.1%; p < 0.025, χ2 test).
In our study, the number of transferred embryos was not the same in all transfers. Only one embryo was transferred in 30.2%, two in 60.0% and three in 9.8% of the cases (Table 2). During the studied period, single embryo transfer was not a predefined policy in the centre. In most cases, only one embryo was transferred because no more embryos were available as demonstrated by the lack of frozen embryo in this group (2.5% of attempts). Moreover, the decision to transfer 3 embryos was mainly motivated by the age of the woman and/or the number of previous attempts without pregnancy and/or the embryo quality. Therefore, the characteristics of populations and embryo quality differed according to the number of transferred embryos (Table 2). The mean age of the women and the mean number of previous IVF attempts were significantly higher when 1 or 3 embryos were transferred. Early or late embryos and fragmented embryos were significantly more frequent, when only one embryo was transferred. Therefore in these cases, the percentage of top quality embryos was significantly lower and could explain the significantly decreased implantation and delivery rates when only one embryo was transferred. When 3 embryos were transferred, the implantation rate was also significantly decreased and the pregnancy and delivery rates were lower but not significantly different. The significantly higher loss of gestational sac after implantation in this group could be more related to maternal factors than to embryonic factors (Table 2). So, to determine whether the influence of embryo quality on post implantation development and pregnancy issue could not be explained only by the women’s age and infertility factors, the data were analysed on the subgroup of 1084 transfers where only 2 embryos were transferred. Similar results were found on delivery and birth rates according to embryo morphology. In this subgroup of transfers, there was also a significantly lower LGSR when 4-cell embryos without fragmentation were transferred compared to all other embryos transferred (16.5% vs 33.5% respectively; p < 0.05, χ2 test). Furthermore, the LGSR was also higher after implantation of 4-cell embryos with 1–20% fragmentation compared to LGSR after implantation of 4-cell embryos without fragmentation (23.5% vs 16.5% respectively).
Table 2.
Patients’, transferred embryos characteristics and transfer outcomes according to the number of transferred embryos 2 days after IVF and ICSI
| No. of transferred embryos | P-value | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Number of transfers (%) | 548 (30.2%) | 1084 (60.0%) | 180 (9.8%) | |
| Female age | ||||
| Mean | 35.1 ± 3.9a,b | 32.6 ± 3.6a,c | 37.4 ± 3.2b,c | a,b,cp<0.001 |
| ≥ 38 years (%) | 25.8a,b | 7.2a,c | 53.4b,c | a,b,cp<0.001 |
| Rank of attempts | ||||
| Mean | 2.1 ± 1.3a,b | 1.7 ± 1a,c | 2.7 ± 1.5b,c | a,b,cp<0.001 |
| ≥ 3 attempts (%) | 30.2a,b | 19a,c | 50b,c | a,c,bp<0.001 |
| Main causes of infertility (%) | ||||
| Female | 31.8a | 31.4b | 42.5a, b | a,bp<0.01 |
| Male | 45.3a, b | 54.1a, c | 31.3b, c | a,b,cp<0.001 |
| Mixed male and female | 13.5a | 7.5a, b | 16.8b | a,bp<0.001 |
| Unknown | 9.4 | 7.1 | 9.5 | |
| Sperm source (%) | ||||
| Fresh ejaculated partner | 82.1 | 83.3 | 88.9 | |
| Frozen-thawed ejaculated partner | 6.4 | 8.4 | 2.8 | |
| Testicular or epididymal | 7.8 | 5.9 | 6.9 | |
| Donor | 3.7 | 2.3 | 1.4 | |
| No. of embryo cells | ||||
| 2 (%) | 26.4a,b | 10.9a | 15b | a,cp<0.001;bp<0.01 |
| 3 (%) | 15.6a,b | 3.4a | 1.2b | a,bp<0.001 |
| 4 (%) | 44.6a,b | 82.4a | 82.8b | a,bp<0.001 |
| 5 (%) | 9.4a,b | 2.8a | 0.5b | a,bp<0.001 |
| 6 (%) | 4 | 0.5 | 0.5 | |
| Embryo fragmentation | ||||
| 0 (%) | 19.7a | 32.9a,c | 21.7c | ap<0.001;cp<0.01 |
| 1–20 (%) | 63.7b | 65.5c | 76.2b,c | b,cp<0.01 |
| 21–50 (%) | 12a | 1.4a | 1.6 | ap<0.001 |
| ≥ 51 (%) | 4.6a,b | 0.2a | 0.5b | ap<0.001;bp<0.025 |
| Top embryos * (%) | 10.2a,b | 29.3a,c | 20.5b,c | a,b,cp<0.001 |
| PR (%) | 9.7a,b | 29.7a | 24.4b | a,bp<0.001 |
| IR (%) | 9.5a | 18.6a,b | 9.6b | a,bp<0.001 |
| LGSR (%) | 26.9 | 21.8a | 35.2a | ap<0.05 |
| DR (%) | 7.1a,b | 22.8a | 16.6b | a,bp<0.001 |
| BR (%) | 6.7a | 14.5a,b | 6.5b | a,bp<0.001 |
PR: Clinical Pregnancy Rate; DR: Delivery Rate; IR: Implantation Rate; LGSR: Number of lost gestational sacs/Total number of gestational sacs; BR: Birth Rate.*Top embryos were defined as embryos at 4-cell stage without any fragmentation when transferred at day-2 after IVF or ICSI.
Discussion
Embryo quality is a major determinant of pregnancy and implantation rates after IVF [17, 20]. However, the influence of early embryo quality on embryo development after implantation and pregnancy outcome is poorly known. In this study, we provide evidence that the pregnancy outcome and the ability to give a live birth are also dependent on the embryo cleavage stage and the integrity of blastomeres on the second day of development.
As previously reported, our results confirm that embryos cleaving too quickly or too slowly during the first two days of development have lower chances to implant. We also clearly demonstrate that such embryos have a lower ability to develop after implantation as the LGSR was higher for embryos with abnormal development kinetics (29.8%) compared to 4-cell embryos (22.7%) 2 days after IVF or ICSI. This is in accordance with the findings published in a recent study [21]. The rate of fragmentation also strongly influences post implantation development. We found that 4 out of 8 embryos (50%) that implanted did not reach birth when the incidence of cell fragmentation was over 20% compared to a LGSR of 21.5% when no fragmentation was observed on the second day of development. A low rate of fragmentation (<21%) weakly affects the ability to develop after implantation even if the loss of gestational sac of 4-cell embryos with <21% fragmentation is higher as compared to LGSR of 4-cell embryos without fragmentation. As demonstrated by the works of Van Blerkom et al. [22] and Hardarson et al. [23], fragmentation of early embryos is a dynamic and sometimes temporary phenomenon. However, our results suggest that it can be an indicator of lower developmental ability even after implantation.
No relationship between the abortion rate and the embryo quality defined according to the number of embryo cells, the percentage of fragmentation and the evenness of the cleavage, had been reported previously [9, 18]. However, significant differences in the number of biochemical pregnancies (defined by the only elevation in hCG without any gestational sac) and in the number of early pregnancy loss were shown, with low chances that a pregnancy obtained after the transfer of embryos with >10% fragmentation or with five or fewer blastomeres at day-3 would lead to the birth of a child [18, 21].
In our series, the 4-cell stage embryos with <21% fragmentation had a higher chance of developing after implantation compared to unfragmented embryos with less or more blastomeres at day 2 (24.2% vs 54% of LGSR respectively) confirming that a change in the kinetics of early cleavage is more detrimental for embryo development than blastomere fragmentation.
The differences of pregnancy outcome and birth rate found in this study could not be explained by the changes of culture media since a similar relation between the embryo quality and pregnancy outcome was observed regardless of the culture media. Furthermore, we did not find any difference on the embryo implantation potential, pregnancy outcome and birth rate according to the fertilization method, IVF or ICSI. The impact of the fertilization method, IVF or ICSI, is poorly documented and contradictory results have been published, with either a better pregnancy rate after ICSI than after IVF [11] or no difference between the two methods of fertilization after the transfer of top quality embryo [8, our series]. Additionally, when analysing data according to the number of transferred embryos and the causes of infertility, similar results of the effect of embryo quality on the pregnancy outcome and birth rate were observed (data not shown).
The lower post implantation development of fragmented embryos may be due in part to a higher rate of chromosomal aneuploïdy in blastomeres [24]. Indeed, the embryo fragmentation observed 68 h after fertilization is known to be correlated with chromosomal abnormality rates [24–28]. Similarly, embryos with a slow cleavage rate resulting in fewer than 6 cells 68 h after fertilization, as well as embryos with a cleavage rate in an abnormal time frame, were shown to be often associated with chromosomal abnormalities [24, 25]. Recently it has been suggested that the lower embryo development might also be the consequence of changes of gene expression in fragmented embryos [29]. Blastomere fragmentation may also be associated with a genetic program of cell death [30], but the relevance of apoptosis in fragmented embryos remains controversial [31].
In this study, only the number of blastomeres and the incidence of cell fragmentation were studied. However, other indicators of embryo quality could also influence the chances of pregnancy. The pronuclear morphology [32–36], the oocyte cytoplasmic halo [37, 38], the blastomere morphology and evenness [17, 23, 39], the presence of multinuclear blastomeres [40–42] and the moment of the first cleavage [14, 43] could also influence the pregnancy rates and could be important factors for the selection of embryos for transfer. Although the results are improved by cumulating various factors [44–46] the relative importance of each factor to the probability of implantation and birth is not known. Only the analysis of very large series of homogeneous embryos transfers could evaluate the respective influence of each embryonic characteristics. Such studies are in progress in our center.
Finally, our study provides useful information to evaluate the probability to obtain a pregnancy, embryo development and delivery and to predict more precisely the real chances of birth in a policy of single embryo transfer in a selected population. In our series when two 4-cell embryos without fragmentation were transferred in women who were less than 38 years old and who had ≤1 previous IVF attempts (203 transfers), the clinical pregnancy rate per transfer was 36.4% and the delivery rate was 31.5%. The implantation rate and birth rate were 23.9 and 20.7% respectively. We can therefore speculate that the delivery rate would have been over 20% if only one embryo had been transferred. Indeed, the delivery rate would have been 10% lower after the first cycle of single embryo transfers, but the remaining non transferred embryo could then have been frozen and been transferred later with a good chance of implantation and birth. We can therefore speculate that the overall delivery rate would not be lower after single embryo transfer in such selected population and that the very high multiple delivery rate which had been 31.2% in this subgroup of women in our series, could have been avoided.
In conclusion, our results show that the morphological aspect of the early embryo not only affects the implantation rate but also the pregnancy outcome, with higher chances that the pregnancy could lead to the birth of a child after transfer of 4-cell stage embryo with low fragmentation.
References
- 1.Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–8. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 2.Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod. 1992;7:117–9. doi: 10.1093/oxfordjournals.humrep.a137542. [DOI] [PubMed] [Google Scholar]
- 3.Ebner T, Yaman C, Moser M, Sommergruber M, Polz W, Tews G. Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril. 2001;76:281–5. doi: 10.1016/S0015-0282(01)01904-5. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Maxson WS, Hoffman DI, Ory SJ, Eager S, Dupre J, Lu C. Maximizing pregnancy rates and limiting higher-order multiple conceptions by determining the optimal number of embryos to transfer based on quality. Fertil Steril. 1998;69:650–7. doi: 10.1016/S0015-0282(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Meniru GI, Craft IL. Embryo developmental stage at transfer influences outcome of treatment with intracytoplasmic sperm injection. J Assisted Reproduction Genetics. 1997;14:245–9. doi: 10.1007/BF02765824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coetsier T, Dhont M. Avoiding multiple pregnancies in in-vitro fertilization: who’s afraid of single embryo transfer? Hum Reprod. 1998;13:2663–4. doi: 10.1093/humrep/13.10.2663. [DOI] [PubMed] [Google Scholar]
- 7.De Neubourg D, Mangelschots K, Van Royen E, Vercruyssen M, Ryckaert G, Valkenburg M, Barudy-Vasquez J, Gerris J. Impact of patients’choice for single embryo transfer in the first IVF/ICSI cycle. Hum Reprod. 2002;17:2621–5. doi: 10.1093/humrep/17.10.2621. [DOI] [PubMed] [Google Scholar]
- 8.De Neubourg D, Gerris J, Mangelschots K, Van Royen E, Vercruyssen M, Elseviers M. Single top quality embryo transfer as a model for prediction of early pregnancy outcome. Hum Reprod. 2004;19:1476–9. doi: 10.1093/humrep/deh283. [DOI] [PubMed] [Google Scholar]
- 9.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, Roulier R. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 10.Martikainen H, Tiitinen A, Tomas C, Tapanainen J, Orava M, Tuomivaara L, Vilska S, Hyden-Granskog C, Hovatta O, Finnish ET, Study Group One versus two embryo transfer after IVF and ICSI: a randomized study. Hum Reprod. 2001;16:1900–3. doi: 10.1093/humrep/16.9.1900. [DOI] [PubMed] [Google Scholar]
- 11.Martikainen H, Orava M, Lakkakorpi J, Tuomivaara L. Day 2 elective single embryo transfer in clinical practice: better outcome in ICSI cycles. Hum Reprod. 2004;19:1364–6. doi: 10.1093/humrep/deh197. [DOI] [PubMed] [Google Scholar]
- 12.Tiitinen A, Halttunen M, Harkki P, Vuoristo P, Hyden-Granskog C. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. 2001;16:1140–4. doi: 10.1093/humrep/16.6.1140. [DOI] [PubMed] [Google Scholar]
- 13.Tiitinen A, Hyden-Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction? The value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. 2004;19:2439–41. doi: 10.1093/humrep/deh446. [DOI] [PubMed] [Google Scholar]
- 14.Van Montfoort AP, Dumoulin JC, Kester AD, Evers JL. Early cleavage is a valuable addition to existing embryo selection parameters: a study using single embryo transfers. Hum Reprod. 2004;19:2103–8. doi: 10.1093/humrep/deh385. [DOI] [PubMed] [Google Scholar]
- 15.Van Montfoort AP, Dumoulin JC, Land JA, Coonen E, Derhaag JG, Evers JL. Elective single embryo transfer (eSET) policy in the first three IVF/ICSI treatment cycles. Hum Reprod. 2005;20:433–6. doi: 10.1093/humrep/deh619. [DOI] [PubMed] [Google Scholar]
- 16.Van Montfoort AP, Fiddelers AA, Janssen JM, Derhaag JG, Dirksen CD, Dunselman GA, Land JA, Geraedts JP, Evers JL, Dumoulin JC. In unselected patients, elective single embryo transfer prevents all multiples, but results in significantly lower pregnancy rates compared with double embryo transfer: a randomized controlled trial. Hum Reprod. 2006;21:338–43. doi: 10.1093/humrep/dei359. [DOI] [PubMed] [Google Scholar]
- 17.Van Royen E, Mangelschots K, De Neubourg D, Valkenburg M, Van de Meerssche M, Ryckaert G, Eestermans W, Gerris J. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–9. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 18.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 19.Papageorgiou T, Guibert J, Goffinet F, Patrat C, Fulla Y, Janssens Y, Zorn JR. Percentile curves of serum estradiol levels during controlled ovarian stimulation in 905 cycles stimulated with recombinant FSH show that high estradiol is not detrimental to IVF outcome. Hum Reprod. 2002;17:2846–50. doi: 10.1093/humrep/17.11.2846. [DOI] [PubMed] [Google Scholar]
- 20.Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Van de Meerssche M, Valkenburg M. Prevention of twin pregnancy after in-vitro fertilization or intracytoplasmic sperm injection based on strict embryo criteria: a prospective randomized clinical trial. Hum Reprod. 1999;14:2581–7. doi: 10.1093/humrep/14.10.2581. [DOI] [PubMed] [Google Scholar]
- 21.Hourvitz A, Lerner-Geva L, Elizur SE, Baum M, Levron J, David B, Meirow D, Yaron R, Dor J. Role of embryo quality in predicting early pregnancy loss following assisted reproductive technology. Reprod Biomed Online. 2006;13:504–9. doi: 10.1016/S1472-6483(10)60637-2. [DOI] [PubMed] [Google Scholar]
- 22.Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod. 2001;16:719–29. doi: 10.1093/humrep/16.4.719. [DOI] [PubMed] [Google Scholar]
- 23.Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16:313–8. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 24.Ziebe S, Lundin K, Loft A, Bergh C, Nyboe Andersen A, Selleskog U, Nielsen D, Grondahl C, Kim H, Arce JC, CEMAS II and Study Group FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum Reprod. 2003;18:2575–81. doi: 10.1093/humrep/deg489. [DOI] [PubMed] [Google Scholar]
- 25.Magli MC, Gianaroli L, Ferraretti AP. Chromosomal abnormalities in embryos. Mol Cell Endocrinol. 2001;22(183 Suppl 1):S29–34. doi: 10.1016/S0303-7207(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 26.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–91. [PubMed] [Google Scholar]
- 27.Pellestor F, Dufour MC, Arnal F, Humeau C. Direct assessment of rate of chromosomal abnormalities in grade IV human embryos produced by in-vitro fertilization procedure. Hum Reprod. 1994;9:293–302. doi: 10.1093/oxfordjournals.humrep.a138497. [DOI] [PubMed] [Google Scholar]
- 28.Plachot M, Junca AM, Mandelbaum J, de Grouchy J, Salat-Baroux J, Cohen J. Chromosome investigations in early life. II. Human preimplantation embryos. Hum Reprod. 1987;2:29–35. doi: 10.1093/oxfordjournals.humrep.a136484. [DOI] [PubMed] [Google Scholar]
- 29.Wells D, Bermudez MG, Steuerwald N, Malter HE, Thornhill AR, Cohen J. Association of abnormal morphology and altered gene expression in human preimplantation embryos. Fertil Steril. 2005;84:343–55. doi: 10.1016/j.fertnstert.2005.01.143. [DOI] [PubMed] [Google Scholar]
- 30.Jurisicova A, Antenos M, Varmuza S, Tilly JL, Casper RF. Expression of apoptosis-related genes during human preimplantation embryo development: potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol Hum Reprod. 2003;9:133–41. doi: 10.1093/molehr/gag016. [DOI] [PubMed] [Google Scholar]
- 31.Martinez F, Rienzi L, Iacobelli M, Ubaldi F, Mendoza C, Greco E, Tesarik J. Caspase activity in preimplantation human embryos is not associated with apoptosis. Hum Reprod. 2002;17:1584–90. doi: 10.1093/humrep/17.6.1584. [DOI] [PubMed] [Google Scholar]
- 32.Chen CK, Shen GY, Horng SG, Wang CW, Huang HY, Wang HS, Soong YK. The relationship of pronuclear stage morphology and chromosome status at cleavage stage. J Assist Reprod Genet. 2003;20:413–20. doi: 10.1023/A:1026232625659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kattera S, Chen C. Developmental potential of human pronuclear zygotes in relation to their pronuclear orientation. Hum Reprod. 2004;19:294–9. doi: 10.1093/humrep/deh064. [DOI] [PubMed] [Google Scholar]
- 34.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Relationship between pre-embryo pronuclear morphology (zygote score) and standard day 2 or 3 embryo morphology with regard to assisted reproductive technique outcomes. Fertil Steril. 2005;84:900–9. doi: 10.1016/j.fertnstert.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 36.Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-Bacrie P, Dumont-Hassan M. Embryos with high implantation potential after intracytoplasmic sperm injection can be recognized by a simple, non-invasive examination of pronuclear morphology. Hum Reprod. 2000;15:1396–9. doi: 10.1093/humrep/15.6.1396. [DOI] [PubMed] [Google Scholar]
- 37.Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Wiesinger R, Puchner M, Tews G. Presence, but not type or degree of extension, of a cytoplasmic halo has a significant influence on preimplantation development and implantation behaviour. Hum Reprod. 2003;18:2406–12. doi: 10.1093/humrep/deg452. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig M, Schopper B, Al-Hasani S, Diedrich K. Clinical use of a pronuclear stage score following intracytoplasmic sperm injection: impact on pregnancy rates under the conditions of the German embryo protection law. Hum Reprod. 2000;15:325–9. doi: 10.1093/humrep/15.2.325. [DOI] [PubMed] [Google Scholar]
- 39.Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19:288–93. doi: 10.1093/humrep/deh070. [DOI] [PubMed] [Google Scholar]
- 40.Çiray HN, Karagenç L, Ulug U, Bener F, Bahçeci M. Early cleavage morphology affects the quality and implantation potential of day 3 embryos. Fertil Steril. 2006;85:358–65. doi: 10.1016/j.fertnstert.2005.07.1301. [DOI] [PubMed] [Google Scholar]
- 41.Moriwaki T, Suganuma N, Hayakawa M, Hibi H, Katsumata Y, Oguchi H, Furuhashi M. Embryo evaluation by analysing blastomere nuclei. Hum Reprod. 2004;19:152–6. doi: 10.1093/humrep/deh003. [DOI] [PubMed] [Google Scholar]
- 42.Van Royen E, Mangelschots K, Vercruyssen M, De Neubourg D, Valkenburg M, Ryckaert G, Gerris J. Multinucleation in cleavage stage embryos. Hum Reprod. 2003;18:1062–9. doi: 10.1093/humrep/deg201. [DOI] [PubMed] [Google Scholar]
- 43.Lundin K, Bergh C, Hardarson T. Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum Reprod. 2001;16:2652–7. doi: 10.1093/humrep/16.12.2652. [DOI] [PubMed] [Google Scholar]
- 44.Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15:2190–6. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 45.Hunault CC, Eijkemans MJ, Pieters MH, te Velde ER, Habbema JD, Fauser BC, Macklon NS. A prediction model for selecting patients undergoing in vitro fertilization for elective single embryo transfer. Fertil Steril. 2002;77:725–32. doi: 10.1016/S0015-0282(01)03243-5. [DOI] [PubMed] [Google Scholar]
- 46.Terriou P, Sapin C, Giorgetti C, Hans E, Spach JL, Roulier R. Embryo score is a better predictor of pregnancy than the number of transferred embryos or female age. Fertil Steril. 2001;75:525–31. doi: 10.1016/S0015-0282(00)01741-6. [DOI] [PubMed] [Google Scholar]