Abstract
The α2 adrenergic receptor antagonist yohimbine (YO) is a sympathomimetic drug that crosses the blood-brain barrier after systemic administration. YO promotes increased transmitter release from noradrenergic (NA) axon terminals in the central nucleus of the amygdala (CEA), bed nucleus of the stria terminalis (BST), hypothalamus, and other brain regions implicated in physiological and behavioral responses to stressful and threatening stimuli. YO is potently anxiogenic in humans and experimental animals, including rats. To determine whether direct connections between the CEA and anterolateral group of BST nuclei (algBST) are necessary for YO anxiogenesis in rats, neurotoxic ibotenate lesions of the CEA in one hemisphere and the ipsi- or contralateral algBST were conducted to disrupt CEA-algBST communication uni- or bilaterally. Sham-lesioned controls received microinjections of vehicle into the CEA and algBST. Two weeks later, anxiety-like behavior was assessed in the elevated plus maze (EPMZ) in rats after i.p. saline or YO (1.0 mg/kg). Central ibotenate lesion placement and extent was assessed postmortem in NeuN-immunolabeled tissue sections. The ability of YO to increase anxiety-like behavior in the EPMZ was similarly robust in rats with sham lesions or ipsilateral CEA-algBST lesions. Conversely, YO anxiogenesis in the EPMZ was disrupted in rats with asymmetric lesions designed to bilaterally disconnect the CEA and algBST, whereas neither unilateral nor bilateral disconnecting lesions altered EPMZ behavior in rats after i.p. saline. We conclude that the anxiogenic effects of increased NA signaling in rats after YO require direct CEA-algBST interactions that do not shape EPMZ behavior under baseline conditions.
Keywords: anxiety, noradrenergic, limbic, stress
Introduction
The emotional state of anxiety is characterized by physiological and behavioral arousal coupled with increased avoidance behavior during exposure to novel or otherwise unpredictable stimuli or environments (Gray, 1982). The amygdala and bed nucleus of the stria terminalis (BST) are implicated as critical components of a widely distributed central neural system that regulates anxiety (Hale et al., 2008). Certain subregions of these limbic forebrain structures, i.e., the central nucleus of the amygdala (CEA) and the anterolateral group subnuclei of the BST (algBST), are bidirectionally interconnected (Dong et al., 2001a, Dong et al., 2001b, Dong and Swanson, 2003, 2004, Walker et al., 2009, Bienkowski and Rinaman, 2012). The CEA and algBST are implicated in stress responsiveness and anxiety (Davis et al., 1997, Herman and Cullinan, 1997, Davis, 2002, Walker et al., 2009), and certain aspects of BST-mediated responses appear to depend on inputs from the CEA (Jasnow et al., 2004, Nakagawa et al., 2005, Walker et al., 2009). However, the functional importance of direct CEA-algBST connections in anxiety remains unexplored.
Previous reports indicate that extensive bilateral lesions of the amygdala or BST do not by themselves alter behavior in laboratory tests commonly used to assess anxiety in rats, including the elevated plus maze (EPMZ) (Treit et al., 1993, Möller et al., 1997, Treit et al., 1998, McHugh et al., 2004). However, such lesions can blunt the anxiogenic effects of treatments such as restraint stress (Möller et al., 1997) that increase central noradrenergic (NA) signaling. Increased NA signaling within the amygdala and BST contributes importantly to the full expression of physiological and behavioral responses to emotional and stressful events (Delfs et al., 2000, Cecchi et al., 2002a, Cecchi et al., 2002b, Morilak et al., 2003, Watanabe et al., 2003, Fendt et al., 2005, Morilak et al., 2005, Banihashemi and Rinaman, 2006). Based on available evidence, we propose that the anxiogenic effect of increased NA signaling depends on CEA-algBST connections. NA projections from the hindbrain to the CEA and algBST are activated by stressful and emotive stimuli (Aston-Jones et al., 1999, Dayas et al., 2001, Myers and Rinaman, 2002, Zhu and Onaka, 2002, Bailey et al., 2003, Myers and Rinaman, 2005, Bienkowski and Rinaman, 2008, Rinaman, 2011), and CEA lesions can reduce BST responses to these stimuli (Nakagawa et al., 2005). It is unclear, however, whether direct CEA-algBST interactions are necessary for behavioral responses to anxiogenic stimuli or treatments that increase central NA signaling above typical baseline levels.
Drugs such as yohimbine (YO) also increase central NA signaling and are sufficient to generate symptoms of emotional distress. YO, which readily crosses the blood-brain barrier after systemic administration, antagonizes norepinephrine (NE) at inhibitory metabotropic α2 adrenergic receptors that are located both pre- and postsynaptically, thereby antagonizing the ability of NE to initiate Gi protein-mediated reductions in intracellular cAMP (Szemeredi et al., 1991). In consequence, YO increases neurotransmitter release from NA axon terminals by blocking presynaptic feedback inhibition of release, and simultaneously reduces the ability of NE to exert inhibitory postsynaptic effects while leaving the excitatory effects of NE at postsynaptic α1 and β receptors unopposed. Systemic YO is potently anxiogenic in humans, and is used clinically as a probe to identify abnormal physiological and affective responses to increased NA signaling (Charney et al., 1983, Gurguis et al., 1997). YO also reliably increases anxiety-like behavior in rats, as evidenced by decreased locomotor and exploratory behaviors and reduced time spent within the open arms of the EPMZ (Handley and Mithani, 1984, Pellow et al., 1985, Baldwin et al., 1989, Johnston and File, 1989, Cole et al., 1995, Redfern and Williams, 1995).
Given the information reviewed above, we hypothesized that direct CEA-algBST connections are necessary for the ability of YO to increase anxiety-like behavior in rats during EPMZ exposure. To test this hypothesis, the excitatory neurotoxin ibotenate (IBO) was used to create fiber-sparing lesions of the CEA and algBST within one hemisphere or in opposite hemispheres, followed by analysis of EPMZ behavior in lesioned and sham-lesioned rats after systemic administration of YO or vehicle. Direct CEA-algBST connections are exclusively ipsilateral in rats (Dong et al., 2001a). Therefore, as illustrated in Figure 1, asymmetrical crossed IBO lesions were designed to disrupt CEA-algBST communication in both hemispheres, whereas ipsilateral IBO lesions were designed to disrupt CEA-algBST communication only unilaterally. Both lesion paradigms spare NA and other central inputs to one non-lesioned CEA and one non-lesioned algBST in each animal, and also preserve neural projections from the intact CEA and algBST to other central targets. Thus, we also hypothesized that EPMZ behavior in i.p. saline-treated rats would not be significantly altered by either unilateral or bilateral CEA-algBST disconnecting lesions.
Figure 1.
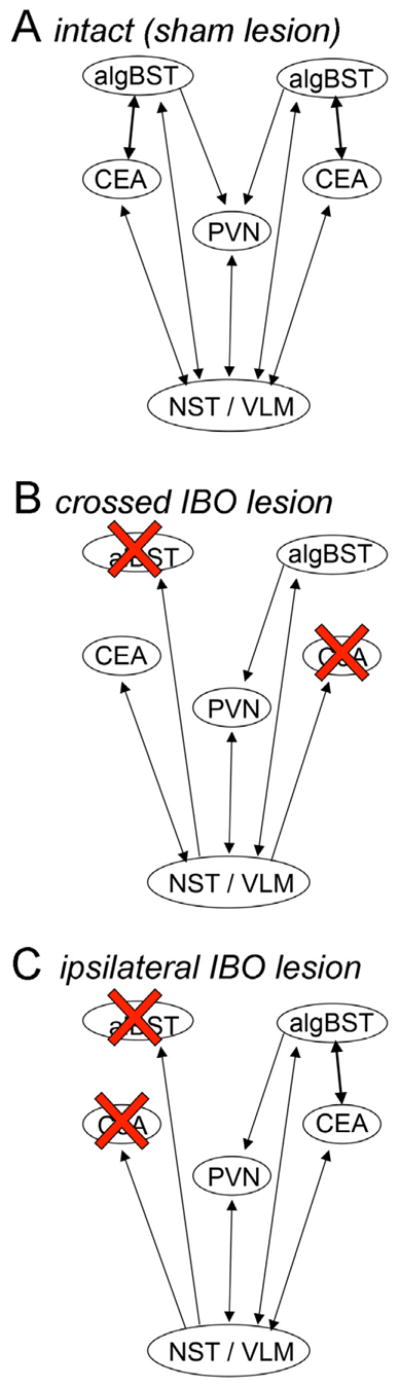
Schematic of connections among the algBST, CEA, paraventricular nucleus of the hypothalamus (PVN), and the hindbrain nucleus of the solitary tract and ventrolateral medulla (NST/VLM). A, in intact (sham lesion) rats, reciprocal connections exist between the algBST and CEA bilaterally. The PVN receives direct input from the algBST bilaterally, and the algBST relays signals from the CEA to the PVN. The PVN, algBST, and CEA each receive direct NA input from the NST and VLM. B, in rats with crossed IBO lesions, the algBST and CEA are disconnected bilaterally, leaving one intact algBST and one intact CEA that do not directly communicate. The PVN and the intact algBST and CEA receive direct NA input from the NST and VLM. The intact algBST projects directly to the PVN, but the algBST no longer relays signals from the CEA to the PVN. C, in rats with ipsilateral IBO lesions, the algBST and CEA are disconnected only in one hemisphere. The PVN receives input from the intact algBST, which relays signals from the intact CEA. The PVN and the intact algBST and CEA receive direct NA input from the NST and VLM. Many other inputs to and outputs from the algBST and CEA are not diagrammed.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (250–275 g BW; Harlan Laboratories) were housed singly in hanging, wire-bottom stainless steel cages in an AAALAC-accredited controlled environment (20–22°C, 12:12 hr light:dark cycle; lights on at 0700 hr) with ad libitum access to water and pelleted chow (Purina 5001). Rats were acclimated to this environment for at least one week before stereotaxic surgery, described below. Experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and are consistent with the U.S. Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Data from 68 rats are included in this report.
Stereotaxic surgery
IBO was microinjected into the CEA on one side of the brain and, in the same rats, into the contralateral or ipsilateral algBST (Fig. 1). Lesion placement was based on results from published tracing studies (Dong et al., 2001a, Shin et al., 2008, Walker et al., 2009), including a recent study from our laboratory that mapped the distribution of CEA neurons that project to the algBST, and of algBSTneurons that project to the CEA (Bienkowski and Rinaman, 2012).
Rats were anesthetized by isoflurane inhalation (1–2% in oxygen) and mounted into a stereotaxic frame in the flat-skull position. A 1.0 μl Hamilton syringe with a sharpened beveled tip was attached to the stereotaxic arm. Injection coordinates targeting the anterolateral subnucleus of the BST (alBST; 0.3 mm caudal, 2.0 mm lateral, and 6.3 mm ventral to bregma) were selected based on a standard rat brain atlas (Swanson, 2004). The alBST is part of the algBST, which also includes the juxtacapsular, oval, rhomboid, fusiform, and subcommissural BST nuclei (Dong and Swanson, 2004). Results from pilot studies indicated that most of the algBST was effectively lesioned by targeting the alBST with an 800 nl infusion of IBO. The injection needle was angled 10 degrees from vertical to avoid passing through the lateral ventricle and septum en route to the alBST target site. IBO was dissolved in vehicle (phosphate buffered saline, pH 7.7) to yield a 0.4% solution. With the beveled tip facing laterally to reduce exposure of the medial BST to injectate, IBO or vehicle (800 nl) was pressure injected into the alBST at 50 nl/min using an automated syringe pump (Stoelting, Wood Dale, IL). The tip was left in place for 5 min after volume delivery. After left side alBST injections, two CEA microinjections of either IBO or vehicle were made on the right side of the brain (crossed IBO lesions) or again on the left side of the brain (ipsilateral IBO lesions) in the same surgical session (500 nl per CEA injection site; 50 nl/min). Injection coordinates targeting the caudal CEA (2.5 mm caudal, 4.2 mm lateral, 8.2 mm ventral to bregma) and the more rostral CEA (1.5 mm caudal, 3.5 mm lateral, 8.2 mm ventral to bregma) were selected based on a standard rat brain atlas (Swanson, 2004). CEA injections were made with the beveled microinjection tip facing medially to reduce exposure of the lateral and basolateral nuclei to injectate. After both CEA injections were complete, the skin was closed with stainless steel clips and an analgesic was administered (Ketoprofen, 2 mg/kg s.c.; Fort Dodge Labs). Rats were returned to their home cages after recovery from anesthesia, where they remained for 2 weeks before EPMZ behavioral data were collected.
EPMZ test
The potential effects of IBO lesions on EPMZ behavior were assessed in rats 2 weeks post-surgery. The EPMZ was constructed according to standard specifications, with four white laminate arms (10 × 50 cm each) oriented in a square cross formation elevated 100 cm above the floor. Two opposing arms were enclosed on three sides by black walls (50 cm tall); the other two arms had no walls but had a continuous plexiglass rim (1 cm tall) to help prevent rats from falling off the open arms during exploration. Thirty minutes before the EPMZ test, IBO- or sham-lesioned rats were injected i.p. with 0.15M NaCl (saline) vehicle (0.7 ml/100 g BW) or with the same volume of saline containing YO (see Fig. 3A for final subgroup sample sizes). YO (Sigma, St. Louis, MO) was freshly dissolved before each experiment by vortexing a measured amount of drug (1.0 mg/kg BW) in sterile 0.15 M NaCl for 5 min at room temperature, followed by passage through a 0.45 μm syringe filter (Millex HV) to remove undissolved residual particles, as previously described (Myers et al., 2005, Banihashemi and Rinaman, 2006). After filtration, the actual YO dose delivered was something less than 1.0 mg/kg BW, but was consistent across rats.
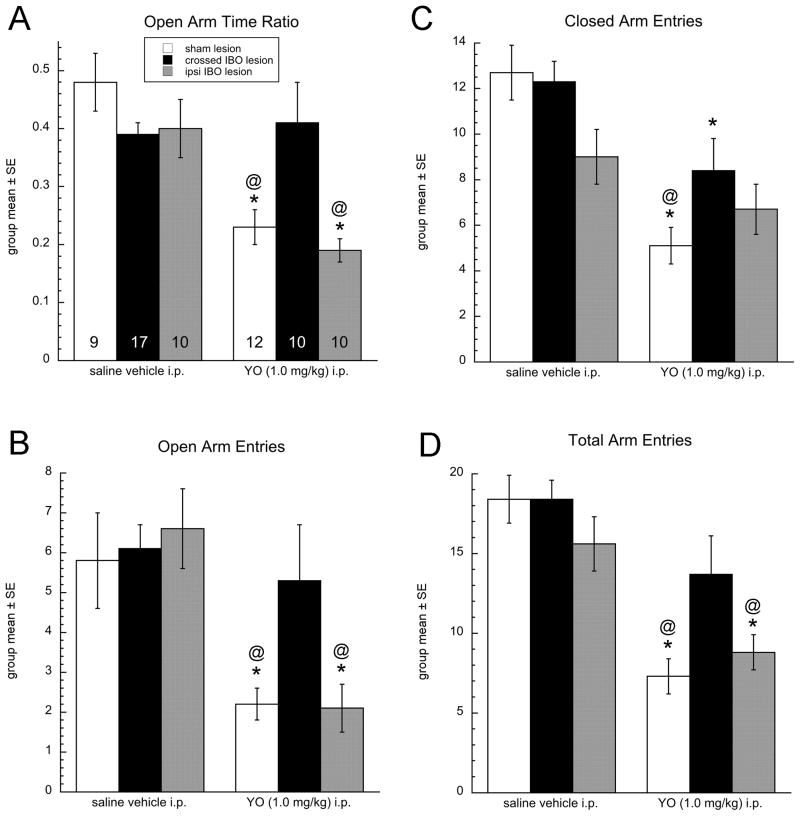
Figure 3.
Behavioral data from the EPMZ test. Surgical group key is boxed in panel A, applies also to B–D. Numbers within the base of each bar in panel A indicate group size (n), and apply also to B-D. A, time spent in the open arms of the maze expressed as open arm ratio (i.e., open arm time/300 sec). B, number of entries into the open arms of the maze. C, number of entries into the closed arms of the maze. D, total number of arm entries. In all panels, * = significantly reduced compared to the same surgical group after i.p. saline, and @ = significantly reduced compared to YO-treated rats with crossed IBO lesions.
Rats were returned to their home cage immediately after i.p. injection. Thirty minutes later (between 0800 and 1000 hr), rats were brought individually into the adjacent testing room and were placed into the center of the EPMZ with their head facing an open arm. The experimenter left the room and the rat was allowed to explore the EPMZ for 5 min (300 sec), with behavior videotaped for later offline analysis. Lighting was moderate and indirect, with both open arms evenly illuminated. The EPMZ was cleaned with a dilute germicidal agent (Quatricide PV) and dried thoroughly between rats.
EPMZ behavioral scoring and data analysis
Videotaped EPMZ behavior was scored offline without knowledge of each rat’s surgical group or i.p. treatment. Scored variables during the 5 min (300 sec) test included the number of open, closed, and total arm entries as a measure of exploratory and locomotor behavior, and cumulative time spent within the open arms as a measure of anxiety-like behavior. The latter was expressed as open arm time ratio for each rat (i.e., seconds spent in open arms/300). Multivariate two-way ANOVA was used to reveal potential interactions and main effects of surgical group (i.e., sham lesioned, crossed IBO lesioned, and ipsilateral IBO lesioned) and i.p. treatment (i.e., YO vs. saline vehicle) on arm entries and open arm time ratio. When F values indicated significant interactions or main effects of surgical group or i.p. treatment, the ANOVA was followed by one-way ANOVAs within surgical group or i.p. treatment group, and post hoc LSD t-test comparisons between groups of interest. Differences were considered statistically significant when P < 0.05. Three rats were considered outliers because their EPMZ open arm time ratios lay more than 2.0 standard deviations away from their respective group means. Data from outlier rats are not included in the results and do not contribute to the reported group sizes.
Perfusion fixation and tissue collection
Approximately one week after EPMZ testing, rats were deeply anesthetized with pentobarbital sodium (Nembutal; 75 mg/kg BW, i.p) and transcardially perfused with aqueous 0.15M NaCl followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Fixed brains were removed from the skull, postfixed for 24 hours, cryoprotected in 20% sucrose, frozen, and sectioned coronally (35 μm) on a freezing-stage microtome. Sections were collected serially in six adjacent sets and either processed immediately as described below (Immunocytochemical procedures), or stored in cyropreservant solution (Watson et al., 1986) at −20°C for later processing.
NeuN immunoperoxidase labeling
One set of tissue sections (i.e., containing sections spaced by 210 μm) from each rat was processed for immunoperoxidase localization of the specific neuronal marker NeuN (Wolf et al., 1996) to identify IBO lesion placement and extent. For this purpose, tissue sections were rinsed for 30 min in 0.1M phosphate buffer followed by 20 min incubation in buffer containing 1% sodium borohydride, 30 min rinsing, 10 min incubation in buffer containing 0.5% H2O2, and 30 min rinsing. Primary and secondary antisera were diluted in buffer containing 1% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.5% Triton X-100 (Sigma). Sections were incubated overnight at room temperature in monoclonal mouse anti-NeuN (1:10,000; Millipore), rinsed, incubated for 90 min in biotinylated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch), rinsed, incubated for 2 hrs in Elite Vectastain avidin-biotin kit reagents (Vector Laboratories, Burlingame, CA), rinsed, and finally reacted for 5 min in 0.1M Na-acetate buffer containing 1% nickel sulfate, 0.5% diaminobenzidine (DAB), and 0.025% H2O2 to generate a blue-black immunoperoxidase reaction product identifying Reacted tissue sections were rinsed, mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), dehydrated in graded alcohols, cleared in xylene, and coverslipped using Cytoseal 60 (VWR, West Chester, PA).
Analysis of IBO lesion placement and extent
Forebrain sections processed for NeuN labeling were examined to reveal regions of neural loss after IBO microinjections into the CEA and alBST. IBO lesion sites were characterized by clear and complete absence of NeuN labeling. A Nikon light microscope connected to a computerized data acquisition system (StereoInvestigator; MBF Bioscience) was used to draw the outer borders of 10–15 tissue sections (spaced 210 μm apart) that spanned the visible extent of NeuN loss in each rat, which typically included sections approximately 3.0 mm caudal to bregma (for CEA lesions) through more rostral sections at the level of bregma or slightly rostral to bregma (for algBST lesions). Regions in which NeuN labeling was absent were plotted onto these mapped sections as “lesion zones”, with reference to a stereotaxic atlas (Swanson, 2004) to approximate the anatomical boundaries of IBO-induced lesions in each rat.
Results
IBO lesion placement
IBO microinjection sites were reliably characterized by a loss of NeuN immunolabeling that defined the boundaries of the lesion (Fig. 2B,D). As expected, lesion size and placement varied across cases. In some IBO rats, large portions of the intended CEA and/or algBST target sites remained NeuN-positive, either due to misdirected IBO injections or apparent failure of IBO neurotoxicity. Cases with misplaced or incomplete lesions were excluded from further analysis and do not contribute to any of the group sizes or data reported in this manuscript. In the remaining IBO-lesioned rats (n=47; 27 crossed IBO and 20 ipsilateral IBO), most of the intended CEA and algBST target regions were characterized by substantial loss of NeuN labeling that often extended into closely adjacent regions (see Fig. 2B,D). EPMZ data from all of these cases were analyzed and are included in the results. It was not possible to generate complete or nearly complete IBO lesions of the CEA or algBST that did not also extend, to varying degrees, into closely adjacent nuclei. However, the common feature across all IBO lesions included in our report is damage to known interconnected regions of the CEA and algBST in the opposite or the same hemisphere. Occasional unilateral damage to small portions of adjacent regions is unlikely to have contributed significantly to experimental outcomes, given that ipsilateral CEA-algBST lesions produced a similar degree of “bystander” damage to adjacent nuclei, but exerted no significant effect on YO anxiogenesis (see Results).
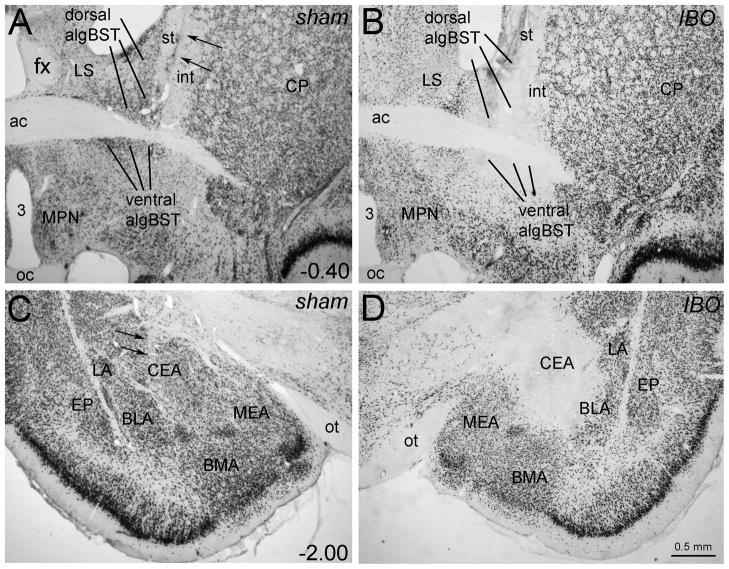
Figure 2.
Representative photomicrographs of NeuN immunoperoxidase-labeled tissue sections from one crossed sham-lesioned rat (A,C) and one ipsilateral IBO-lesioned rat (B,D). Two small arrows point out injection needle tracts in the sham-lesioned rat (A: algBST target site; C: CEA target site), although there is no visible loss of NeuN labeling. Conversely, the IBO-lesioned rat displays a restricted loss of NeuN immunolabeling within the dorsal and ventral algBST (B) and the CEA (D). The algBST lesion site depicted in panel B extends partially into the subjacent LPO, while the CEA lesion site depicted in panel D extends partially into the closely adjacent BLA. Scale bar in D applies also to A-C. Abbreviations not defined in manuscript: 3, third ventricle; ac, anterior commissure; BMA, basomedial amygdala; CP, caudate putamen; EP, endopiriform nucleus; fx, fornix; int, internal capsule; LA, lateral amygdala; LPO, lateral preoptic area; LS, lateral septum; MEA, medial amygdala; MPN, medial preoptic nucleus; oc, optic chiasm; ot, optic tract; st, stria terminalis.
The needle tract could usually be located in tissue sections from sham-lesioned rats that received vehicle microinjections into the CEA and the contralateral or ipsilateral algBST. However, it was not possible to identify vehicle microinjection sites, as there was no apparent loss of NeuN-positive neurons (Fig. 2A,C). EPMZ data from all sham-lesioned rats (n=21; 15 crossed sham and 6 ipsilateral sham) are included in the results.
EPMZ behavior
Among sham-lesioned control rats, EPMZ behavior did not vary significantly as a function of stereotaxic injection placement (i.e., crossed vs. ipsilateral vehicle injections into the algBST and CEA), and so data from sham-lesioned rats were grouped together according to i.p. treatment (n=9 saline vehicle, n=12 YO; see Figure 3).
Multivariate two-way ANOVA revealed a main effect of i.p. treatment (saline vs. YO) on all assessed parameters, a main effect of surgical group (sham vs. ipsi IBO vs. crossed IBO lesion) on maze open arm time and total arm entries, and an interaction between i.p. treatment and surgical group on open arm time (Table 1). Graphical results and post-hoc t-comparisons are illustrated in Figure 3. As expected, YO markedly reduced open arm time and the number of arm entries (open, closed, and total) in sham-lesioned rats. Interestingly, among rats tested in the EPMZ after i.p. saline, surgical lesion group had no significant effect on any dependent behavioral variable [Fig. 3; F(2,35)=0.18–3.11 across variables, all P values > 0.05]. Conversely, in YO-treated rats, surgical group had a significant effect on open arm time ratio [F(2,31)=6.36, P=0.005], open arm entries [F(2,31)=4.61, P=0.018], and total arm entries [F(2,31)=4.42, P=0.021], but not closed arm entries (P=0.12). Post hoc tests confirmed that open arm time ratio, open arm entries, and total arm entries were reduced to a similar extent by YO in rats with sham or ipsilateral IBO lesions, whereas the same behavioral parameters were not reduced by YO treatment in rats with asymmetrical crossed IBO lesions (Fig. 3).
Table 1.
Multivariate two-way ANOVA main effects, interactions, and P values for EPMZ behavioral parameters in sham-lesioned, crossed IBO-lesioned, and ipsilateral IBO-lesioned rats after i.p. saline or YO.
| Effect Source | EPMZ parameter | F | P < |
|---|---|---|---|
| I.p. treatment [F(1,62)] Saline vehicle vs. YO (1.0 mg/kg) |
Open arm time ratio | 18.6 | * 0.001 |
| Open arm entries | 16.7 | * 0.001 | |
| Closed arm entries | 25.5 | * 0.001 | |
| Total entries | 35.2 | * 0.001 | |
|
| |||
| Surgical group [F(2,62)] Sham vs. crossed IBO vs. ipsi IBO lesioned |
Open arm time ratio | 3.3 | * 0.04 |
| Open arm entries | 2.2 | n.s. (P=0.12) | |
| Closed arm entries | 2.7 | n.s. (P=0.08) | |
| Total entries | 3.7 | * 0.03 | |
|
| |||
| Interaction [F(2,62)] Surgical group x i.p. treatment |
Open arm time ratio | 6.4 | * 0.003 |
| Open arm entries | 2.6 | n.s. (P=0.08) | |
| Closed arm entries | 2.8 | n.s. (P=0.07) | |
| Total entries | 2.3 | n.s. (P=0.11) | |
See Figure 3 for graphical display of data and post-hoc comparisons. ns, not significant.
Discussion
Results from previous studies indicate that increased NA signaling within the CEA and algBST promotes anxiety-like behavior in rats (Cecchi et al., 2002b; Cecchi et al., 2002a). The present study used an asymmetrical disconnecting lesion approach and EPMZ testing to determine whether direct CEA-algBST interactions are necessary for the behavioral effects of increased NA signaling after YO treatment. Consistent with previous reports (Pellow et al., 1985, Baldwin et al., 1989, Johnston and File, 1989, Cole et al., 1995), systemic administration of YO in sham-lesioned rats reduced their overall maze exploration and reduced the proportion of total test time spent within the open arms of the maze. Ipsilateral IBO lesions that disrupted CEA-algBST interactions in only one hemisphere did not significantly alter the ability of YO to reduce maze exploration or increase anxiety-like behavior, and neither asymmetrical crossed nor ipsilateral IBO lesions altered EPMZ behavior in rats after i.p. saline. Conversely, crossed IBO lesions that disrupted CEA-algBST interactions in both hemispheres significantly disrupted the ability of systemic YO to decrease maze exploration and open arm time. These results support the view that CEA-algBST communication in at least one hemisphere is necessary for the ability of YO to increase anxiety-like maze behavior in rats, whereas disruption of CEA-algBST communication has no significant effect on maze behavior under conditions in which central NA signaling is not pharmacologically enhanced.
Among adrenergic receptors, YO is specific in its antagonism of the α2 “inhibitory” subtype, and was used in this study as an experimental tool to increase excitatory NA receptor signaling. Although YO has been suggested or reported to interact with receptors for other monoamines (Johnston and File, 1989, Winter and Rabin, 1992, Millan et al., 2000), the α2 receptor agonist clonidine reverses YO-induced anxiety, and the ability of YO to increase cFos expression in the cortex, amygdala, and other brain regions in rats is attenuated or blocked by pretreatment with clonidine or propranolol, a postsynaptic β-adrenergic receptor antagonist (Charney et al., 1983, Gubits et al., 1989, Johnston and File, 1989, Bing et al., 1992, Tsujino et al., 1992). Nevertheless, interpretation of our results does not depend on whether or not YO’s actions are limited to increased transmitter release from NA terminals and increased postsynaptic adrenergic receptor signaling. Instead, YO can be viewed as an effective pharmacological tool that reliably promotes an organismic state of sympathetic arousal and increased anxiety, and that the latter effect depends on CEA-algBST interactions.
The CEA and BST receive direct and relayed input from sensory and cortical areas that transmit the occurrence of stressful or emotional events (Swanson, 2000, Watts and Swanson, 2002), and both regions provide output to hypothalamic and brainstem nuclei that drive behavioral, hormonal, and autonomic responses to these events (LeDoux et al., 1988, Herman and Cullinan, 1997, Swanson and Petrovich, 1998). Previous investigators have used electrolytic or neurotoxic approaches to inactivate or lesion amygdala and BST regions that include the CEA and algBST, to examine effects on stress-related emotional behaviors. For example, CEA inactivation blocks fear-potentiated but not light-enhanced startle (Walker and Davis, 1997), attenuates conditioned freezing, and abolishes conditioned place avoidance (CPA) responses to shock-paired cues and contexts (Holahan and White, 2004a, b). CEA lesions and algBST lesions are similarly effective in blocking the ability of a noxious visceral stimulus to support CPA (Tanimoto et al., 2003, Deyama et al., 2007), supporting roles for both regions in negative affective responses to visceral feedback. The BST is necessary for the ability of morphine withdrawal to support CPA, an effect which also requires an intact CEA (Nakagawa et al., 2005). Bilateral CEA lesions that reduce hypothalamic-pituitary-adrenal (HPA) axis activation in a cytokine model of interoceptive stress (i.e., systemic interleukin-1β) also significantly reduced cFos activation in the algBST (Xu et al., 1999). It is important to note that the studies cited above used bilateral lesions or inactivation of either the CEA or BST to achieve significant experimental outcomes; thus, in addition to removing CEA or BST contributions to the assessed outcome, CEA-algBST communication was also bilaterally disrupted. However, although extensive bilateral lesions of the amygdala or BST disrupt CEA-algBST circuits bilaterally, they do not by themselves alter baseline behavior in the EPMZ or other tests commonly used to assess anxiety-like behavior in rats (Treit et al., 1993, Möller et al., 1997, Treit et al., 1998, McHugh et al., 2004). One report indicates that bilateral neurotoxic BNST lesions attenuate baseline anxiety-like behavior in rats as assessed in the elevated zero maze (Waddell et al., 2006), although that study used female rats that had previously been exposed to a shock conditioning procedure, which might have recruited central NA signaling that to exert an anxiogenic effect on “baseline” maze performance.
CEA lesions can blunt the anxiogenic effects of treatments such as restraint stress (Möller et al., 1997) that increase central noradrenergic (NA) signaling, consistent with the present results that CEA-algBST circuit disruption blocks the ability of YO to increase anxiety-like behavior in the EPMZ. Our study is the first to examine the functional consequences of disconnecting the CEA from the algBST by using fiber-sparing neurotoxic lesions. One CEA and one algBST were preserved in each IBO-lesioned animal; thus, both asymmetrical crossed and ipsilateral lesions preserved the multiple other inputs to and outputs from one intact CEA and one intact algBST in each rat. Our results demonstrate that asymmetrical crossed IBO lesions completely disrupt YO anxiogenesis, whereas ipsilateral lesions do not. The asymmetrical crossed IBO lesion also removed a key route through which the CEA modulates both algBST and hypothalamic neural responses to stressful and emotional events (see Fig. 1). In contrast, ipsilateral IBO lesions did not attenuate the ability of YO to reduce maze exploration and reduce open arm time ratio. Thus, in the absence of direct CEA-algBST communication in at least one hemisphere, the multiple other inputs and outputs of the intact CEA and the intact algBST are insufficient to support YO anxiogenesis in the EPMZ. By extension, CEA-algBST interactions may exhibit varying degrees of necessity for mediating behavioral and physiological responses to naturalistic threats that increase central NA signaling.
As part of the “striatal” amygdala, CEA neurons are primarily GABAergic and co-express CRF, similar to neurons in the algBST (Swanson and Petrovich, 1998, Dong et al., 2001a). Thus, CEA-algBST communication is mediated largely through GABAergic and CRF signaling, and there is good evidence that CRF receptor signaling within the BST plays a critical role in anxiety and other behavioral responses to threatening stimuli (Erb et al., 2001, Jasnow et al., 2004, Walker et al., 2009). The similar maze behavior displayed by rats in all three lesion groups after i.p. saline supports the view that GABAergic/CRF-mediated connections between the CEA and algBST are not critical for shaping exploratory behavior in the EPMZ in the absence of YO, despite the apparent necessity of these connections for anxiogenic responses to increased NA signaling after YO treatment. This conclusion is consistent with a previous report that pharmacological blockade of postsynaptic adrenoceptors within the anterior BST does not alter baseline EPMZ behavior, although it does reverse the anxiogenic effects of prior immobilization stress (Cecchi et al., 2002a). We propose that CEA-algBST circuits are engaged by YO and other stimuli that increase central NA signaling in such a way as to shift behavior towards anxiogenesis. This proposal is ripe for future studies using other experimental paradigms and assays to explore the circuit features and necessity of CEA-algBST interactions for mediating behavioral and physiological responses to actual and perceived homeostatic threats.
Highlights.
The central amygdala and lateral bed nucleus are directly connected.
Disconnecting lesions did not alter baseline behavior in the elevated plus maze (EPMZ).
Bilateral disconnecting lesions blocked yohimbine anxiogenesis in the EPMZ.
EPMZ responses to increased noradrenergic signaling require intra-limbic connections.
Acknowledgments
The authors thank Michael Bienkowski for helpful discussions on limbic circuitry. Research supported by the National Institutes of Health (MH59911).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? Journal of Psychopharmacology. 2003;17:252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Johnston AL, File SE. Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. European Journal of Pharmacology. 1989;159:211–215. doi: 10.1016/0014-2999(89)90709-7. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and the anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Structure & Function. 2012 doi: 10.1007/s00429-012-0393-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing C, Stone EA, Zhang Y, Filer D. Immunohistochemical studies of noradrenergic-induced expression of c-fos in the rat CNS. Brain Research. 1992;592:57–62. doi: 10.1016/0006-8993(92)91658-2. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002a;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002b;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DEJ. Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Science. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology. 1995;121:118–126. doi: 10.1007/BF02245598. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural circuitry of anxiety and stress disorders. In: Davis KL, et al., editors. Neuropsychopharmacology: the Fifth Generation of Progress. American College of Neuropsychopharmacology; 2002. pp. 931–951. [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. European Journal of Neuroscience. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deyama S, Nakagawa T, Kaneko S, Uehara T, Minami M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behavioural Brain Research. 2007;176:367–371. doi: 10.1016/j.bbr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: Implications for cerebral hemisphere regulation of ingestive behaviors. The Journal of Comparative Neurology. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. The Journal of Neuroscience. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Clarendon Press; 1982. [Google Scholar]
- Gubits RM, Smith TM, Fairhurst JL, Yu H. Adrenergic receptors mediate changes in c-fos mRNA levels in brain. Brain Research Molecular Brain Research. 1989;6:39–45. doi: 10.1016/0169-328x(89)90026-0. [DOI] [PubMed] [Google Scholar]
- Gurguis GNM, Vitton BJ, Uhde TW. Behavioral, sympathetic and adrenocortical responses to yohimbine in panic disorder patients and normal controls. Psychiatry Research. 1997;71:27–39. doi: 10.1016/s0165-1781(97)00041-3. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenergic agonists and antagonists in a maze-exploration model of “fear”-motivated behaviour. Naunyn-Schmied Archives of Pharmacology. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holahan MR, White NM. Amygdala inactivation blocks expression of conditioned memory modulation and the promotion of avoidance and freezing. Behavioral Neuroscience. 2004a;118:24–35. doi: 10.1037/0735-7044.118.1.24. [DOI] [PubMed] [Google Scholar]
- Holahan MR, White NM. Intra-amygdala muscimol injections impair freezing and place avoidance in aversive contextual conditioning. Learning and Memory. 2004b;11:436–446. doi: 10.1101/lm.64704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral Neuroscience. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Yohimbine’s anxiogenic action: Evidence for noradrenergic and dopaminergic sites. Pharmacology Biochemistry and Behavior. 1989;32:151–156. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Deacon RMJ, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas J-P, Cogé F, Galizzi J-P, Boutin JA, Rivet J-M, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at α2-adrenergic receptors (AR)s, serotonin (5-HT)1A, 5-HT1B, 5-HT1D and dopamine D2 and D3 receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Research. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Progress in Neuro- Pschopharmacology and Biological Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sciences. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. The Journal of Comparative Neurology. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiology and Behavior. 2002;77:723–729. doi: 10.1016/s0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L. Trimethylthiazoline (TMT) supports conditioned flavor avoidance and activates viscerosensory, hypothalamic, and limbic circuits in rats. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2005;288:R1716–R1726. doi: 10.1152/ajpregu.00479.2004. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Redfern W, Williams A. A re-evaluation of the role of alpha 2-adrenoceptors in the anxiogenic effects of yohimbine, using the selective antagonist delequamine in the rat. British Journal of Pharmacology. 1995;116:2081–2089. doi: 10.1111/j.1476-5381.1995.tb16415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-W, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. Structure of the Rat Brain. Amsterdam: Elsevier; 2004. Brain Maps III. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neuroscience. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Szemeredi K, Komoly S, Kopin IJ, Bagdy G, Keiser HR, Goldstein DS. Simultaneous measurement of plasma and brain extracellular fluid concentrations of catechols after yohimbine administration in rats. Brain Research. 1991;542:8–13. doi: 10.1016/0006-8993(91)90990-d. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pain in rats. European Journal of Neuroscience. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behavioral Neuroscience. 1998;112:379–386. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- Treit D, Pesold C, Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behavioral Neuroscience. 1993;107:770–785. doi: 10.1037//0735-7044.107.5.770. [DOI] [PubMed] [Google Scholar]
- Tsujino T, Sano H, Kubota Y, Hsieh S-T, Miyajima T, Saito K, Nakajima M, Saito N, Yokoyama M. Expression of Fos-like immunoreactivity by yohimbine and clonidine in the rat brain. European Journal of Pharmacology. 1992;226:69–78. doi: 10.1016/0922-4106(92)90084-9. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology. 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand ST, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Anatomy of motivational systems. In: Gallistel R, editor. Stevens’ Handbook of Experimental Psychology. New York: John Wiley & Sons; 2002. pp. 563–631. [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. Journal of Pharmacological and Experimental Therapeutics. 1992;263:682–689. [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I. NeuN: a useful neuronal marker for diagnostic histopathology. Journal of Histochemistry & Cytochemistry. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1b administration. Neuroscience. 1999;94:175–183. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- Zhu L, Onaka T. Involvement of medullary A2 noradrenergic neurons in the activation of oxytocin neurons after conditioned fear stimuli. European Journal of Neuroscience. 2002;16:2186–2198. doi: 10.1046/j.1460-9568.2002.02285.x. [DOI] [PubMed] [Google Scholar]