Abstract
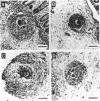
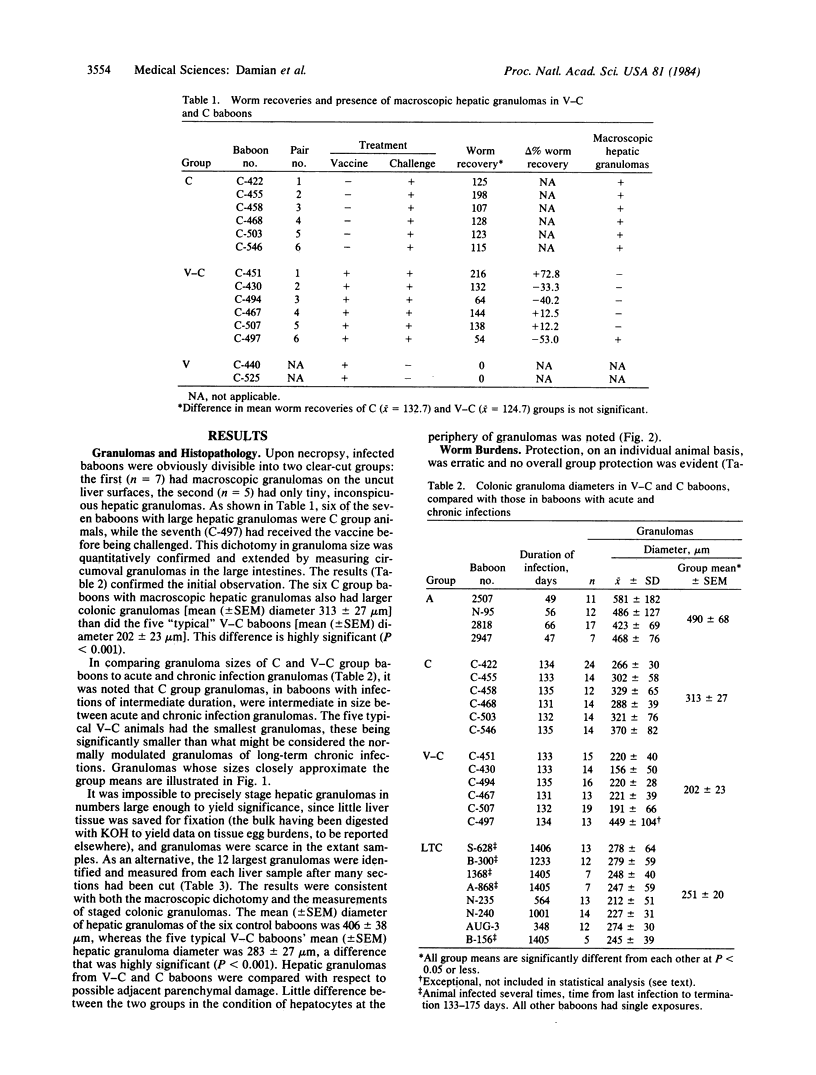
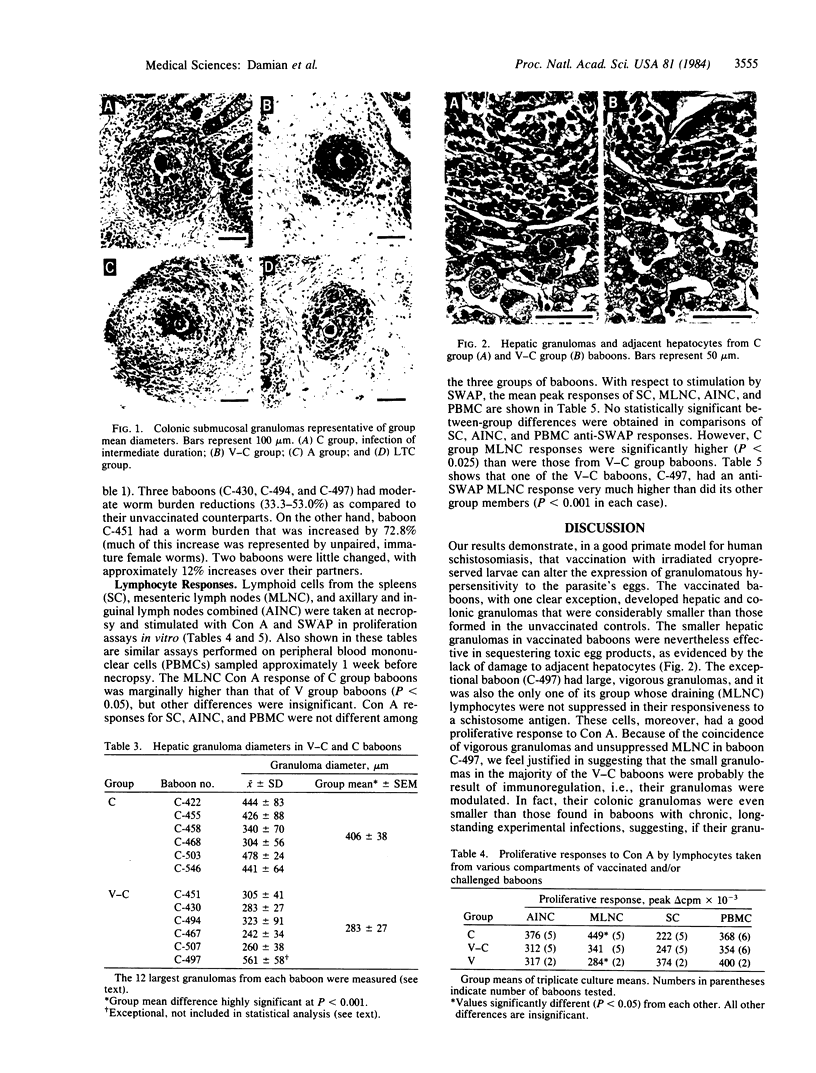
Young male baboons born in captivity were immunized with an attenuated, cryopreserved schistosomular vaccine derived from gamma-irradiated (50 krad) cercariae of the Puerto Rican strain of Schistosoma mansoni. Protection against a heterologous Kenyan strain of S. mansoni, after percutaneous infection, was assessed. Partial protection (33-53% reduction in worm burden) was obtained in three of six vaccinated-challenged baboons, an unremarkable result. Of greater interest was the fact that all six unvaccinated-challenged control baboons, but only one of the six vaccinated-challenged baboons, had macroscopic egg granulomas on their liver surfaces. This difference in granuloma size was substantiated by measuring hepatic and colonic granulomas. The mean (+/- SEM) hepatic and colonic granuloma diameters for the six unvaccinated baboons were 406 +/- 38 micron and 313 +/- 27 micron, respectively, and for the five "typical" vaccinated-challenged baboons the mean diameters were 283 +/- 27 micron and 202 +/- 23 micron, respectively. Both hepatic and colonic granulomas were significantly smaller in the five typical vaccinated-challenged baboons. Not only did the exceptional vaccinated-challenged baboon have very large hepatic and colonic granulomas, but also it was the only one of its group whose mesenteric lymph node cells were not suppressed in their in vitro proliferative response to a schistosome antigen. These results strongly suggest that granuloma size reduction in the majority of the vaccinated baboons was the result of immunoregulation--i.e., the small postvaccination granulomas were "modulated." Despite their small size, hepatic granulomas in the typical vaccinated baboons were apparently as effective in sequestering egg toxins and preventing hepatocyte damage as the larger granulomas of the control baboons. Smaller, less obstructive granulomas are thought to be more beneficial to the host than large, vigorous granulomas, with respect to lessening chronic disease. The present results give encouragement that a vaccine to ameliorate disease in human schistosomiasis is possible. This effect should add to the attractiveness of partial protection against challenge infections conferred by attenuated larval vaccines, as reported by others, to yield a dually beneficial vaccine for human use.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE Z. A. WARREN KS: MILD PROLONGED SCHISTOSOMIASIS IN MICE: ALTERATIONS IN HOST RESPONSE WITH TIME AND THE DEVELOPMENT OF PORTAL FIBROSIS. Trans R Soc Trop Med Hyg. 1964 Jan;58:53–57. doi: 10.1016/0035-9203(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Damian R. T., Greene N. D., Fitzgerald K. Schistosomiasis mansoni in baboons. The effect of surgical transfer of adult Schistosoma mansoni upon subsequent challenge infection. Am J Trop Med Hyg. 1972 Nov;21(6):951–958. [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Fanning M. M., Peters P. A., Davis R. S., Kazura J. W., Mahmoud A. A. Immunopathology of murine infection with Schistosoma mansoni: relationship of genetic background to hepatosplenic disease and modulation. J Infect Dis. 1981 Aug;144(2):148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- Green W. F., Colley D. G. Modulation of Schistosoma mansoni egg-induced granuloma formation: I-J restriction of T cell-mediated suppression in a chronic parasitic infection. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1152–1156. doi: 10.1073/pnas.78.2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L. M., Boros D. L., Warren K. S. Induction of immunological hyporesponsiveness to granulomatous hypersensitivity in Schistosoma mansoni infection. J Infect Dis. 1974 Nov;130(5):515–522. doi: 10.1093/infdis/130.5.515. [DOI] [PubMed] [Google Scholar]
- Hillyer G. V., Cangiano J. L. Schistosoma mansoni granuloma in immunosuppressed man. Report of a case. Trans R Soc Trop Med Hyg. 1979;73(3):331–333. doi: 10.1016/0035-9203(79)90095-6. [DOI] [PubMed] [Google Scholar]
- Jordan P., Von Lichtenberg F., Goatly K. D. Experimental schistosomiasis in primates in Tanzania. Preliminary observations on the susceptibility of the baboon Papio anubis to Schistosoma haematobium and Schistosoma mansoni. Bull World Health Organ. 1967;37(3):393–403. [PMC free article] [PubMed] [Google Scholar]
- Lewis F. A., Stirewalt M., Leef J. L. Schistosoma mansoni: radiation dose and morphologic integrity of schistosomules as factors for an effective cryopreserved live vaccine. Am J Trop Med Hyg. 1984 Jan;33(1):125–131. doi: 10.4269/ajtmh.1984.33.125. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Garcia E. G., Cruise K. M., Tiu W. U., Hocking R. E. Lung granulomatous hypersensitivity to eggs of Schistosoma japonicum in mice analysed by a radioisotopic assay and effects of hybridoma (idiotype) sensitization. Aust J Exp Biol Med Sci. 1982 Aug;60(Pt 4):401–416. doi: 10.1038/icb.1982.45. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Brown A. P., Warren K. S., Pelley R. P., Houba V., Siongok T. K., Ouma J., Sturrock R. F., Butterworth A. E. Factors that modify the cellular-immune response in patients infected by Schistosoma mansoni. J Immunol. 1980 Nov;125(5):1916–1923. [PubMed] [Google Scholar]
- Sadun E. H., Von Lichtenberg F., Bruce J. I. Susceptibility and comparative pathology of ten species of primates exposed to infection with Schistosoma mansoni. Am J Trop Med Hyg. 1966 Sep;15(5):705–718. doi: 10.4269/ajtmh.1966.15.705. [DOI] [PubMed] [Google Scholar]
- Stek M F. r., Minard P., Dean D. A., Hall J. E. Immunization of Baboons with Schistosoma mansoni Cercariae attenuated by gamma irradiation. Science. 1981 Jun 26;212(4502):1518–1520. doi: 10.1126/science.7233238. [DOI] [PubMed] [Google Scholar]
- Stirewalt M. A., Cousin C. E., Dorsey C. H. Schistosoma mansoni: stimulus and transformation of cercariae into schistosomules. Exp Parasitol. 1983 Dec;56(3):358–368. doi: 10.1016/0014-4894(83)90081-4. [DOI] [PubMed] [Google Scholar]
- Stirewalt M. A., Lewis F. A., Murrell K. D. Schistosoma mansoni: cryopreservation of schistosomules. Exp Parasitol. 1979 Oct;48(2):272–281. doi: 10.1016/0014-4894(79)90109-7. [DOI] [PubMed] [Google Scholar]
- Stirewalt M., Lewis F. A., Cousin C. E., Leef J. L. Cryopreservation of schistosomules of Schistosoma mansoni in quantity. Am J Trop Med Hyg. 1984 Jan;33(1):116–124. doi: 10.4269/ajtmh.1984.33.116. [DOI] [PubMed] [Google Scholar]
- Sturrock R. F., Butterworth A. E., Houba V. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology. 1976 Dec;73(3):239–252. doi: 10.1017/s003118200004693x. [DOI] [PubMed] [Google Scholar]
- Taylor M. G., James E. R., Nelson G. S., Bickle Q., Andrews B. J., Dobinson A. R., Webbe G. Immunisation of baboons against Schistosoma mansoni using irradiated S. mansoni cercariae and schistosomula and non-irradiated S. rodhaini cercariae. J Helminthol. 1976 Sep;50(3):215–221. doi: 10.1017/s0022149x00027772. [DOI] [PubMed] [Google Scholar]
- Taylor M. G., Nelson G. S., Smith M., Andrews B. J. Studies on heterologous immunity in schistosomiasis. 7. Observations on the development of acquired homologous and heterologous immunity to Schistosoma mansoni in baboons. Bull World Health Organ. 1973;49(1):57–65. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Schistosoma mansoni: stage specificity of granuloma formation around eggs after exposure to irradiated cercariae, unisexual infections, or dead worms. Exp Parasitol. 1970 Feb;27(1):60–66. doi: 10.1016/s0014-4894(70)80010-8. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972;66(3):417–434. doi: 10.1016/0035-9203(72)90273-8. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The secret of the immunopathogenesis of schistosomiasis: in vivo models. Immunol Rev. 1982;61:189–213. doi: 10.1111/j.1600-065x.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Webbe G., Sturrock R. F., James E. R., James C. Schistosoma haematobium in the baboon (Papio anubis): effect of vaccination with irradiated larvae on the subsequent infection with percutaneously applied cercariae. Trans R Soc Trop Med Hyg. 1982;76(3):354–361. doi: 10.1016/0035-9203(82)90189-4. [DOI] [PubMed] [Google Scholar]
- Weinstock J. V., Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. V. Participation of histamine receptor positive and negative lymphocytes in the granulomatous response of Schistosoma mansoni-infected mice. J Immunol. 1983 Jan;130(1):423–427. [PubMed] [Google Scholar]