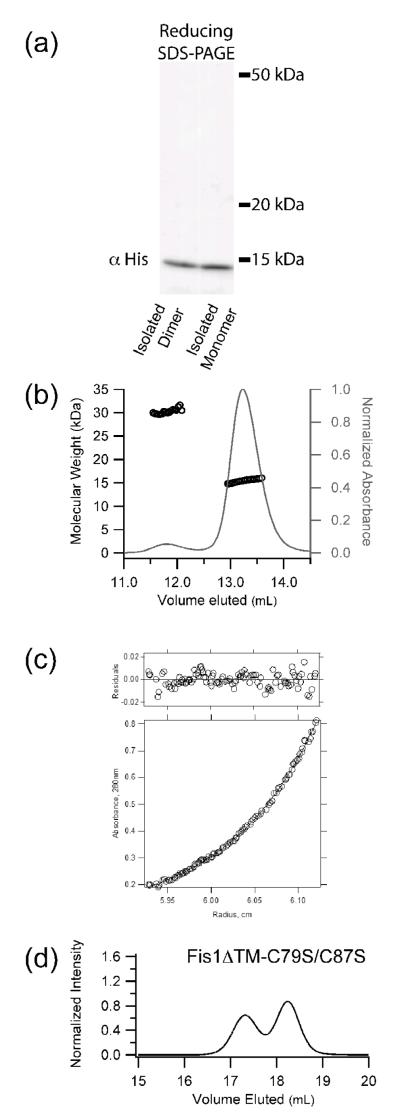
Figure 2. The wild type cytoplasmic domain of Fis1 (Fis1ΔTM) forms a noncovalent dimer.
a) Recombinant yeast Fis1ΔTM was isolated as two species by size exclusion chromatography, diluted to 2.5 μM, analyzed by non-reducing SDS-PAGE, and detected by Western blot analysis. b) Fis1ΔTM (1.3 mg/mL) eluted by size exclusion chromatography and detected by multi-angle laser light scattering has a calculated molar masses of 30,300 and 15,500 Da, which compares favorably with the theoretical molecular weights for dimer and monomer (33,400 and 16,700 Da, respectively). c) The higher order species of yeast Fis1ΔTM is a dimer. A radial distribution fit to a monomer-dimer equilibrium model is shown for a sample at 23.2 μM centrifuged at 20,000 rpm. The residuals of the fit are shown in the top panel. These data are representative of a global analysis of Fis1ΔTM samples enriched in the higher order species that were subjected to equilibrium sedimentation analysis at 16300, 20000, 24500 and 30000 rpm at 4 °C on a Beckman XLA ultracentrifuge. Samples were at 7.7 μM, 15.4 μM and 23.2 μM in 50 mM sodium phosphate and 184 mM NaCl, pH 7.4. A global fit to the data was well described by a model in which the higher order species of Fis1ΔTM forms a dimer. d) Size exclusion chromatography of a cysteine-less variant of recombinantly expressed Fis1ΔTM-C79S/C87S (2.5 μM) eluted as both monomer and dimer. Experimental conditions identical to Figure 1. Both Cys residues lie on helix 4 of the concave surface.