Abstract
The study of diabetic cardiomyopathy (diabetic CM) is an area of significant interest given the strong association between diabetes and the risk of heart failure. Many unanswered questions remain regarding the clinical definition and pathogenesis of this metabolic cardiomyopathy. This article reviews the current understanding of diabetic CM with a particular emphasis on the unresolved issues that have limited translation of scientific discovery to patient bedside.
Keywords: diabetic cardiomyopathy, mitochondria, heart failure, metabolism
Case Vignette
A 58-year male with no past medical history presents with 3 months of progressive dyspnea on exertion and mild LE edema. On initial evaluation his hemoglobin A1c is elevated at 7.8%, fasting triglycerides are increased at 220 mg/dl, and his HDL is low at 30 mg/dl. He undergoes an echocardiogram which reveals moderate LVH, low normal EF (45%), pseudonormal diastolic filling, and a dilated IVC. He has mild, diffuse coronary artery disease on cardiac catheterization and his blood pressure is 135/80.
What is the most likely etiology of his heart failure?
How does diabetes influence cardiac metabolism and function?
How should his diabetes be treated given his cardiomyopathy?
1. Introduction
The prevalence of obesity in the United States has reached epidemic proportions. As a consequence, obesity related diseases, such as diabetes, also continue to increase at a staggering rate. Cardiovascular complications are common in diabetics and account for the majority of morbidity and mortality in this population. In particular, the link between diabetes and heart failure (HF) has gained increased attention over the past several decades. The term “diabetic cardiomyopathy” (diabetic CM) was first coined in the early 1970’s by Rubler, who identified 4 patients at autopsy with diabetic nephrosclerosis and a non-ischemic cardiomyopathy 1. Since that time epidemiologic studies have confirmed that diabetics are more than twice as likely to develop HF compared to non-diabetics 2. Moreover, the survival of diabetic HF patients is also reduced relative to those without diabetes 3. For these reasons, understanding the pathogenic mechanisms responsible for diabetic myocardial disease is of significant interest.
The accepted clinical definition of diabetic CM is the presence of diastolic or systolic cardiac dysfunction in a diabetic patient without other obvious causes for cardiomyopathy, such as coronary artery disease (CAD), hypertension (HTN), or valvular heart disease. Given the vague nature of this definition and lack of true diagnostic criteria, diabetic CM remains a somewhat elusive entity. However, extensive clinical and animal model research has identified certain structural and pathologic findings that characterize this metabolic cardiomyopathy. Typically, left ventricular hypertrophy (LVH) and diastolic dysfunction are the earliest manifestations of diabetic CM, with systolic dysfunction occurring later in the course of disease. However, given the loose clinical criterion for diagnosing diabetic CM, there is some uncertainty as to its natural history.
The strong association between diabetes and HF has fueled intense human and animal research aimed at identifying the mechanisms underlying diabetic myocardial disease. Several pathologic abnormalities have been identified in the diabetic heart including myocardial lipid overload, altered substrate utilization, oxidative stress, fibrosis, inflammation, and mitochondrial dysfunction. Although significant progress has been made, the precise underpinnings of diabetic CM remain controversial. In fact, many still question whether diabetes in and of itself is capable of producing overt HF. In this chapter, we will discuss the current thinking with regards to the pathogenesis and management of diabetic CM, with an emphasis on areas of uncertainty. In addition, the interplay between diabetes and other HF risk factors will be discussed.
2. Pathogenesis
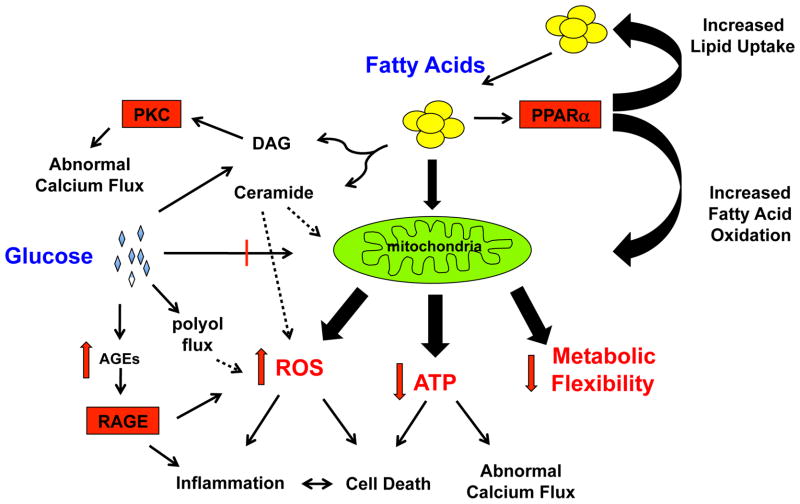
The pathogenesis of diabetic CM is complex and multifactorial (Fig. 1). However, several common themes have emerged. This section will focus first on the structural and functional abnormalities that occur in the diabetic heart, and then review the potential molecular mechanisms contributing to myocyte dysfunction.
Figure 1. The multifaceted effects of diabetes on cardiomyocyte biology.
In the diabetic state, excess fatty acids are present inside the cell leading to excessive mitochondrial FAO, PPARα activation, and the generation of lipid signaling molecules such as ceramide and DAG. This metabolic reprogramming leads to mitochondrial dysfunction manifested by excess ROS production, less efficient ATP generation, metabolic inflexibility. Stressed mitochondria also amplify inflammatory and cell death responses. Hyperglycemia can further augment cardiac myocyte toxicity via the formation of AGEs as well as promoting excess ROS and DAG generation.
2.1 Structural and functional characterization of Diabetic CM
LVH
LVH is a significant predictor for the development of heart failure, and is associated with increased mortality 4, 5. Although hypertension is the leading risk factor for the development of LVH, substantial evidence indicates that diabetes can also trigger this pathologic remodeling response. Echocardiographic studies performed in diabetic patients have consistently shown a strong association between diabetes, increased LV mass, and LVH even in the absence of coexistent HTN 6, 7. Moreover, obesity itself also portends an increased risk of concentric LVH independent of elevated blood pressures 8. Consistent with this observation, there is evidence to suggest that adipose tissue derived cytokines may contribute to cardiac hypertrophy in situations of nutrient excess 9. Moreover, hyperinsulinemia may also contribute to cardiac myocyte hypertrophy 10. Although the precise mechanisms of the hypertrophic response to metabolic stress remain to be fully elucidated, LVH has become a defining structural characteristic of diabetic CM.
Diastolic Dysfunction
LVH and hypertrophic remodeling are associated with abnormal myocardial relaxation and diastolic dysfunction. Similar to the data surrounding LVH in metabolic disease, there is strong link between diabetes and diastolic dysfunction. In fact, diastolic abnormalities are thought to be amongst the earliest functional manifestations of diabetic CM. The prevalence of diastolic dysfunction in diabetics ranges between 40–75% 11, 12. Moreover, the majority of type 1 and type 2 diabetic animal models also reveal diastolic function abnormalities by echocardiography and pressure-volume loop analysis 13. Importantly, diastolic dysfunction is apparent in these models even in the absence of HTN or CAD. The mechanism of diastolic dysfunction in the diabetic heart may be a consequence of abnormal calcium handling, impaired energetics, cardiac lipid accumulation, and/or myocardial fibrosis. Interestingly, the early stages of diastolic dysfunction are reversible in diabetics who lose weight and normalize their metabolism 14. This finding implies that the pathogenesis of diabetic CM may have a reversible phase and emphasizes the importance of early, aggressive lifestyle modification in diabetics with impaired myocardial relaxation.
Systolic Dysfunction
Reduced LV systolic function is generally considered to be a late manifestation of diabetic CM. It is unknown whether systolic HF is the final common pathway of diabetic CM or is an alternate phenotype determined by the interaction between genetics and diabetes in susceptible individuals. It is also important to recognize that many diabetics with “normal” ejection fraction may actually have impaired systolic function when more sophisticated measures, such as myocardial strain measurements or tissue doppler, are employed 15. As such, the early stages of systolic dysfunction are likely to go unrecognized clinically. Similar to the general population, the presence of systolic HF is associated with worse prognosis in patients with diabetes.
2.2 Cellular and Molecular Defects in Diabetic CM
Glucotoxicity
Hyperglycemia has long been considered a central component in the pathogenesis of diabetic CM. In part, this is supported by evidence that poor blood glucose control correlates with increased risk of developing HF in diabetic patients 16. In general, hyperglycemia is thought to contribute to cardiac dysfunction through two potential mechanisms: 1) the generation of advanced glycation end products (AGEs) and/or 2) the induction of oxidative stress.
Elevated blood glucose levels can lead to the non-enzymatic post-translational modification of proteins, lipids, and nucleic acids. Following rearrangement to the more stable amadori products these modified molecules are referred to as AGEs 17. Not surprisingly, the level of AGEs in serum and tissue are significantly elevated in patients with diabetes 18. In addition to impairing the function of modified proteins or nucleic acids, AGEs can trigger biologic responses via the receptor for AGEs, or RAGE. RAGE is a member of the immunoglobulin superfamily that is expressed on a wide variety of cells including macrophages, cardiac myoctyes, endothelial cells, and smooth muscle cells 19. RAGE signaling leads to the activation of MAP kinases, PI-3 Kinase, rho GTPases, NF-κB, and NADPH oxidase which triggers inflammatory cytokine production and reactive oxygen species (ROS) generation. Evidence supporting a role for RAGE activation in the pathogenesis of cardiac dysfunction in diabetes was recently published using a streptozotocin (STZ)-induced model of type 1 diabetes in rats. In this study, treatment of diabetic rats with aminoguanidine, a small molecule that prevents AGE formation, reduced ventricular and vascular stiffening20. However, aminoguanidine also has RAGE-independent effects that may be relevant to this phenotype. There is still debate about the importance of AGE/RAGE signaling in diabetic CM as most of the data comes from animal models of profound and untreated hyperglycemia. Thus, additional research will be necessary to address this question.
Hyperglycemia has also been linked to increased oxidative stress independent of RAGE. The mechanism of ROS generation in this instance can occur via increased glucose flux through the polyol pathway, hexosamine pathway, and mitochrondrial oxidative phosphorylation 21. A more detailed discussion of oxidative stress in the pathogenesis of diabetic CM is presented below.
Given the dogma that hyperglycemia drives diabetic cardiovascular disease, it is surprising that clinical studies have failed to show that aggressive blood glucose control reduces the incidence of cardiovascular complications in diabetics22, 23. Although this may reflect an inadequate understanding of how best to lower blood glucose, an alternative explanation is that other metabolic factors contribute to the pathogenesis of diabetic myocardial disease independent of hyperglycemia. Potential candidates are altered lipid metabolism, inflammation, and mitochondrial dysfunction. These factors and their sequela will be discussed in the following sections.
Altered lipid metabolism
The heart is a metabolic omnivore that is capable of using diverse substrates for ATP generation. Under normal conditions the heart generates ~ 70% of its ATP from fatty acid oxidation (FAO), with glucose oxidation contributing most of the remainder 24. Substrate flexibility is also a hallmark of cardiac metabolism. To maintain adequate ATP generation in the face of physiologic or metabolic stress the heart must be able shift substrate utilization. This metabolic flexibility is thought to play a crucial role in protecting cardiac myocytes from injury when ATP demand increases and/or substrate availability decreases.
The impact of obesity and diabetes on myocardial metabolism has been well studied over the past 2 decades. In diabetes, the heart is bathed in elevated concentrations of fatty acids and glucose. Human studies using positron emission tomography tracers have reproducibly demonstrated increased basal FAO and reduced glucose oxidation rates in patients with obesity and diabetes 25, 26. Consistent with these findings, in vivo and ex vivo animal models have also shown that type 1 and type 2 diabetes lead to increased myocardial FAO capacity 27, 28 As observed in human studies, animal models also confirm that both glycolysis and glucose oxidation are reduced in the diabetic myocardium 29. In part, this is a reflection of myocardial insulin resistance 30. Thus, despite the presence of elevated glucose in the extracellular environment, its uptake and utilization is impaired in the diabetic heart.
Metabolic reprogramming in the diabetic heart includes transcriptional and post-transcriptional mechanisms. The PPARα gene expression network is induced in hearts from diabetic animals and humans based on mRNA and protein analysis 31, 32. PPARα is a nuclear receptor transcription factor whose activity is regulated by fatty acid ligands. The gene targets of PPARα are involved in fatty acid import, FAO, and triglyceride synthesis 33. In addition, PPARα can suppress glucose oxidation via the transcriptional induction of PDK4, which prevents the entry of glucose into the citric acid cycle 34. PPARα-independent mechanisms also contribute to the metabolic shift in the diabetic myocardium, including reduced expression of the insulin responsive glucose transporter GLUT4 35. As a consequence of reduced myocardial glucose uptake, FAO rates increase to maintain constant levels of cellular ATP.
The augmentation of FAO in diabetes is likely an adaptive mechanism in the short term, designed to handle excessive fatty acid delivery and reduced glucose availability. However, over time this response becomes maladaptive and can potentially lead to myocyte dysfunction. The mechanisms by which altered fatty acid metabolism contributes to cardiac dysfunction is an area of active research, but may include excessive mitochondrial ROS production, less efficient energy generation, and the production of incompletely oxidized acyl-carnatine metabolites and/toxic lipid species 36. Metabolic reprogramming of the diabetic heart also reduces its substrate flexibility. This is relevant in times of stress when the inability to utilize more efficient substrates, such as glucose, could lead to myocyte energy depletion and dysfunction. There are no clinically available therapies for HF targeted towards modulating myocardial metabolism, making this an attractive area for continued research and clinical translation.
Lipotoxicity
The presence of cardiac myocyte steatosis is a well-established pathologic hallmark of diabetic CM. The accumulation of lipid may seem paradoxical in the setting of increased fatty acid utilization, but this observation reflects the imbalance between FA uptake and oxidation that occurs in the diabetic heart. Autopsy studies of patients with non-ischemic cardiomyopathy have revealed that diabetic patients have a significantly more neutral lipid within cardiac myocytes compared to non-diabetics 37. Consistent with these observations, MRI based quantification of myocardial triglyceride has also demonstrated that insulin resistance and diabetes are associated with a significant increase in cardiac lipid content 38, 39. Cardiac steatosis is also readily observed in animal models of diabetes, arguing that this is a defining characteristic of diabetic CM 40. Of note, the accumulation of lipid appears to precede the onset of cardiac dysfunction.
There is a growing body of evidence that supports a role for myocardial lipid accumulation in the pathogenesis of diabetic CM. Several transgenic mouse models of cardiac steatosis have demonstrated that lipid overload can promote LVH and cardiomyopathy in the absence of systemic metabolic perturbations such as insulin resistance and hyperglycemia 31, 41–43. The mechanism(s) by which lipids promotes cardiac toxicity is not clear, but animal model and cell culture data have implicated ER stress, ceramide accumulation, oxidative stress, and mitochondrial dysfunction 44, 45. Moreover, lipid remodeling of ER and mitochondrial membranes may also be important in the pathogenesis of lipotoxicity. Further investigation with non-transgenic animal models will be required to determine the importance of lipotoxicity to the phenotype of diabetic CM.
Oxidative Stress
Increased oxidative stress is another common theme in models of diabetic CM. As discussed above, animal and human data demonstrates increased ROS in the diabetic myocardium. Oxidative stress appears to correlate with excess lipid delivery and elevated mitochondrial FAO rates, arguing that mitochondria are an important source of free radicals in the diabetic heart 40. However, high rates of FAO do not always lead to excessive ROS generation, implying other derangements in mitochondrial structure and function must also be involved 46. Hyperglycemia can also trigger oxidative stress via mitochondrial and non-mitochondrial glucose metabolic pathways 47. Enzymatic sources of ROS such as that generated by the NAPDH oxidase complex, which is induced by RAGE activation, may also be important for the redox environment in diabetic cardiomyoctyes. In support of this notion, it was recently published that the NAPDH oxidase system is activated in the diabetic heart 48. Dysfunctional ROS scavenging mechanisms have also been proposed to contribute to the severity of oxidative stress in the diabetic heart 49. More than likely, a combination of these mechanisms is responsible for the ROS observed in diabetic CM.
The functional importance of oxidative stress in diabetic CM has also been investigated. Mechanistically, it has been postulated that ROS can cause contractile dysfunction through damage of intracellular organelles and proteins. In support of this concept, overexpression of antioxidants such as superoxide dismutase, catalase, metallothionein, and glutathione peroxidase significantly improved contractile function in ex vivo hearts and cardiac myocytes from diabetic mice 50–53. Despite these promising results, most of the data to date comes from STZ models of diabetic heart failure. Whether these findings will translate to other diabetic animal models and humans remains to be determined. Nonetheless, the consistent finding of increased oxidant stress in diabetic CM warrants additional research to define the pathologic consequences of excess ROS on cardiac function and to explore the optimal means of reducing this oxidative stress.
Abnormal calcium handling
Cardiac myocyte calcium handling is known to play a key role in the regulation of myocardial contraction and relaxation. In systole, L-type calcium channels allow the influx of calcium which triggers calcium mediated calcium release from the SR. The mobilization of SR calcium is mediated by the ryanodine sensivie (RyR) calcium channel. During diastole, calcium must be re-sequestered into the SR to allow for cardiac myocyte relaxation. The SERCA2a channel is necessary for this to occur. In diabetes, both the RyR and SERCA channels are dysregulated. The RyR channel is downregulated and hyperphosphorylated in models of both type 1 and type 2 diabetes 54–56. Hyperphosphylation leads to increased calcium leak from the RyR receptor, thereby depleting SR calcium and increasing cytoplasmic calcium during diastole. At the same time, SERCA2a activity is reduced in the diabetic state, further exacerbating SR calcium depletion and impairing calcium sequestration during diastole 57. In combination, these changes in calcium flux impair both systolic contractility and diastolic relaxation. Interestingly, both oxidative stress and mitochondrial dysfunction have been implicated in impaired calcium flux 58.
PKC signaling
Several protein kinase C (PKC) isoforms are hyperactivated in the diabetic myocardium 59. The regulation of PKC activity occurs via the lipid signaling molecule diacylglyerol (DAG). In the diabetic heart, DAG levels are elevated as a consequence of enhanced angiotensin II and catacholemine mediated activation of phospholipase C, the enzyme which cleaves phosphatidylinositol 4,5-bisphosphate to form DAG 60. In addition, de novo synthesis of DAG is augmented in states of glucose and fatty acid excess, such as occurs in diabetic cardiomyocytes.
Hyperactive PKC signaling in the heart can influence calcium handling, ROS generation, and inflammation all of which can affect cardiac performance. In support of this notion, transgenic mice that overexpress PKCβ in cardiac myocytes develop cardiomyopathy 61. There is also evidence that PKCβ inhibition can improve the cardiac phenotype of STZ-injected rats 62. Future investigation will be needed to determine the utility of PKC modulation in other models of diabetic myocardial disease.
Apoptosis/inflammation/fibrosis
Inflammation, cell damage/death, and fibrosis are also pathologic hallmarks of diabetic CM. Inflammation is now recognized as a key participant in the pathogenesis of diabetes and its complications 63. In models of type 1 and type 2 diabetes the expression of inflammatory cytokines such as TNFα and IL-6 is increased in the myocardium 64, 65. A modest increase in macrophages and monocytes has been described in the diabetic myocardium, but the role of these cells in diabetic CM has not been well studied. The initial trigger for metabolic inflammation may be ER stress pathways, ROS, and/or the release of danger associated molecular patterns (DAMPS) released from damaged myocytes. Interestingly, inhibition of TNF or caspase 1 (responsible for IL-1β production) reduced myocardial inflammation and improved cardiac function in a rat model of STZ-induced diabetes 64, 66. Thus, targeting specific inflammatory pathways may be a novel therapeutic approach for the treatment. Future research investigating the initiation and consequences of metabolic inflammation in the diabetic heart is needed. In particular, defining the molecular mechanisms of crosstralk between myocytes and cardiac leukocytes is of interest.
Increased myocyte necrosis and apoptosis is seen in animal and human diabetic hearts 67–69. Although the mechanism of cell death remains unclear, impaired energetics, oxidative stress, inflammatory cytokines and fatty acid–induced lipotoxicity have all been implicated in this response 70. The loss of cardiac myocytes in diabetic CM could contribute directly to LV systolic dysfunction, but more likely the dying cells and their intracellular contents serve to amplify pro-inflammatory and pro-fibrotic pathways.
Cardiac fibrosis is frequently observed in diabetic hearts and this association is independent of CAD or HTN 71, 72. Both animal model and human tissue samples provide evidence that progressive myocardial fibrosis may be a component of diastolic and systolic dysfunction in diabetic CM. However, it should be noted that significant fibrosis is not a feature in all models of diabetic CM 73. Cardiac myocyte cell death and inflammation can activate pro-fibrotic pathways in the heart. TGF-β is thought to play an important role in this process 74. In addition, the expression and activity of matrix metalloproteinase (MMP) 2 is significantly diminished in STZ-induced diabetic CM, potentially reducing collagen turnover and increasing fibrosis 75–77. In combination, increased production and reduced degradation of collagen may contribute to the pathogenesis of the progressive fibrosis observed in diabetic CM.
Mitochondrial dysfunction
As discussed above, diabetes dramatically alters mitochondrial substrate utilization and oxidative flux. Early in the course of insulin resistance there is an increase in both mitochondrial number and FAO capacity 78, 79. This appears to be an adaptive response designed to handle the increase in lipid delivery and the reduction in glucose import. However, as overt diabetes develops mitochondrial dysfunction becomes apparent. Specifically, there are changes in the morphology, respiratory capacity, and proteome of mitochondria in the diabetic heart 80.
Mitochondrial respiratory function has been studied in numerous diabetic models. In type 2 diabetes, the expression of mitochondrial uncoupling proteins is increased which enhances oxygen consumption and reduces ATP generation during mitochondrial oxidative phosphorylation 81, 82. This results in reduced cardiac efficiency and increased ROS generation. These observations were recently extended to humans where freshly isolated human atrial myocytes from diabetic and non-diabetic patients undergoing CABG were investigated. Consistent with the mouse data, mitochondrial preparations from diabetic myocytes had less efficient ATP generation and increased ROS production compared to non-diabetic samples 83. Together this data argues that mitochondria in the diabetic heart are less able to generate ATP and more likely to trigger oxidative stress in cardiac myocytes. However, whether the diabetic myocardium is truly “energy deficient” is still an area of debate.
It is attractive to consider that abnormalities in mitochondrial biology may be a unifying feature of the multitude of derangements present in the diabetic myocardium. Mitochondria play a key role in metabolic flux/energy production, ROS generation, and inflammation. All of which are core features of diabetic heart failure (Fig. 1). Moreover, alterations in cellular energetics and redox environment likely contribute to many of stress responses observed in the diabetic heart such as cell death and dysregulated calcium handling. Thus, modulating mitochondrial function in the heart has the potential to improve numerous aspects of the diabetic CM phenotype.
2.3 Summary
The last 30 years have witnessed an explosion of research focused on the diabetic heart. As illustrated in the above sections, the impact of diabetes on myocardial biology is complex and multifactorial. Moreover, much of the cardiac functional data in animal models comes from ex vivo assessment of cardiac myocyte performance. In fact, reports of in vivo functional abnormalities in mouse models of type 1 and type 2 diabetes, as determined by echocardiography, have been inconsistent and often underwhelming 73, 84–87. This is further confounded by the large amount of data derived from STZ-induced diabetic models where profound hyperglycemia, volume depletion, tissue atrophy and/or the direct effects of STZ on the myocardium likely contribute to the observed phenotypes 88. In the end, it is still a legitimate question to ask what is diabetic CM? It is clear that the diabetic milieu influences myocardial biology and when pushed to extremes can produce cardiac dysfunction. However, the more clinically relevant issue may be how diabetes modulates the myocardial response to other stressors. This is particularly true in humans, where diabetes frequently co-exists with other HF risk factors. In the next section we will explore this concept further and discuss the interplay between diabetes and other cardiac stress.
3. Diabetes and the vulnerable myocardium
The preceding section focused on the mechanisms by which diabetes, as a single disease, can impact myocardial biology. However, the vast majority of diabetics also have other co-morbidities that can influence cardiac function such as CAD and HTN.
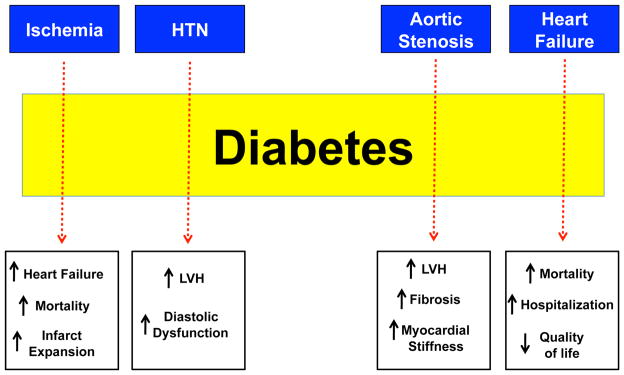
For this reason understanding how diabetes impacts the response of the myocardium to other injurious stimuli is clinically relevant. Strong evidence supports the concept that the diabetic heart is more susceptible to damage inflicted by other stressors (Fig. 2). Thus, diabetes can function as an amplifier cardiac injury. This point is illustrated by data investigating the interaction between diabetes and cardiac damage induced by acute myocardial infarction (AMI) and aortic stenosis (AS).
Figure 2. Diabetes amplifies cardiac injury response to a variety of stimuli.
Diabetes augments the risk of adverse cardiovascular outcomes in patients with ischemic heart disease, HTN, AS, and heart failure (blue boxes). The dashed arrows connecting to the text boxes indicate how diabetes influences the outcomes of the above-mentioned cardiovascular diseases.
3.1 Diabetes and Myocardial Ischemia
Diabetes is associated with accelerated CAD and an increased risk of AMI 89. What is less well appreciated is that diabetes also alters the response of the myocardium to ischemic stress. Clinical data has consistently demonstrated that diabetics have an increased risk of death following acute MI 90–92. This holds true even when the size of the initial infarct is considered. The excess mortality in this patient population is largely due to an increased incidence of post-infarction HF 93–95. These observations highlight the importance of understanding the mechanisms by which diabetes influences the myocardial response to ischemic injury.
Animal models of diabetes and AMI outcomes have replicated the findings of clinical studies. Namely, AMI leads to exaggerated adverse LV remodeling and increased mortality following left anterior descending artery occlusion in type 1 and type 2 diabetic models 96–99. The similarities in cardiac phenotype between diabetic humans and mice following AMI suggest that animal models may be useful to dissect the mechanisms of this phenomenon. The majority of data in this area has been descriptive; however, enhanced myocardial injury following ischemia in diabetes has been proposed to involve dysregulated inflammation, increased oxidative stress, and/or microvascular disease 100. The molecular basis of these responses and whether they will serve as therapeutic targets in the future remains to be determined.
3.2 Diabetes and Aortic Stenosis
In addition to acute ischemic stress, the response of the diabetic myocardium to chronic pressure overload, such as seen with AS, is also abnormal. In a recent study of patients with severe AS, diabetes was associated with increased LV mass and reduced systolic function despite similar aortic value gradients 101. Moreover, this association was independent of co-existing CAD. In a similar study, diabetics with AS had increased myocardial fibrosis as compared to their non-diabetic counterparts 102. Interestingly, diabetes was also associated with increased levels of the hypophosphorylated N2B titin isoform. In sum these changes could significantly increase myocardial stiffness, exacerbate diastolic dysfunction, and worsen HF symptoms. There is still much to learn about how diabetes renders the heart more vulnerable to damage from ischemia and pressure overload. Morever, the impact of diabetes on other myocardial stressors such as myocarditis and genetic stress should be explored
3.3 Diabetes and the progression of heart failure
In addition to being a risk factor for HF, diabetes can also modulate the natural history of this disease. In retrospective analyses of patients with reduced and preserved systolic function HF, diabetes is an independent predictor of rehospitalizations and mortality 103–105. Moreover, diabetes also promotes accelerated adverse myocardial remodeling in the setting of HTN, another important HF risk factor 106. Thus, diabetic myocardial remodeling appears to significantly impact the evolution of HF irrespective of the etiology. Continued investigation into the mechanism(s) of this phenomenon is warranted.
4. Management of Diabetes of HF
There are no clear guidelines for the management of diabetes in HF patients. In large part this a consequence of the paucity of clinical trial data in this area. This section will briefly discuss issues to consider when managing diabetes in patients with HF.
4.1 Insulin replacement strategies
In addition to insulin injections, this group also includes the orally administered sulfonylureas (SU), such as glyburide. In retrospective analyses, the use of insulin for DM was associated with an increased incidence and severity of HF 107, 108. However, given the retrospective, non-randomized nature of these studies, it is not possible to determine whether insulin treatment truly increases of the risk of HF or identifies a higher risk diabetic patient. Similar data is present for the SUs. Moreover, the risk of hypoglycemia is increased with the use of insulin or SUs. In general, these agents should not be used as first line therapy for diabetes in HF patients.
4.2 Insulin sensitizing strategies
The drugs in this class include the biguanides, such as metformin, and the thiozolidendiones (TZDs), such as pioglitazone and rosiglitazone. Metformin was initially contraindicated in patients with HF due to the perceived risk of lactic acidosis. However, more recent observational data argues that the risk of lactic acidosis is very low in this patient population 109. This has led to renewed interest in metformin as a treatment option for patients with HF and diabetes. In a study by Eurich et al, diabetic HF patients treated with metformin alone or in combination with a SU had a significant reduction in mortality compared to patients treated with a SU alone 110. Similar results have also been reported from other retrospective database analyses 13, 111. Although no randomized controlled trial data exists, it appears that metformin treatment is safe in patients with DM and HF, and it may improve outcomes.
The TZDs have been a source of controversy in the field of cardiology. The impact of these medications on CAD is still a topic of debate; however, the initial concerns appear to overstate the risk 112, 113. In 5–10% of patients TZDs will trigger fluid retention, which leads to an increased risk of HF hospitalization in patients with diastolic and/or systolic dysfunction 114. For this reason, many patients with diabetes and HF will not tolerate these medications. Similar to metformin, there is retrospective data that associates TZD-containing regimens with improved mortality in HF patients 115. However, given the lack of clinical trials to prove benefit and the potential harm of increasing hospitalization for HF, TZDs should be used with caution in HF patients. Whether TZDs can reduce the risk of developing HF in patients with diabetics with normal cardiac function remains an unanswered question. In the future, more selective TZDs may produce the desired metabolic effects without the risk of fluid retention.
4.3 Incretin based therapies
Modulation of the incretin system has shown promise as a means to improve blood glucose levels and reduce diabetic complications. Natural incretins, such as glucagon-like peptide-1 (GLP1), are small molecules that are secreted by intestinal epithelial cells in response to food ingestion. GLP-1 mediates its biologic effects via the GLP-1 receptor, which is expressed on a wide variety of cells in the pancreas, heart, lung, kidney and, hypothalamus. In response to GLP-1, glucose-stimulated insulin release from the pancreas is enhanced. The clearance of GLP-1 is extremely rapid and controlled by the enzyme dipeptidyl peptidase 4 (DPP4). The available agents targeting this pathway function either as the GLP-1 receptor agonists (i.e. exentinide/Byetta) or DPP4 inhibitors (sitiglipitin/Januvia).
Evidence supporting the use of incretin-based therapies for reducing cardiovascular complications in diabetes is growing. In an animal model of atherosclerosis, GLP-1 significantly reduced plaque burden 116. In addition to anti-atherogenic effects, the GLP-1 pathway may also have cardioprotective properties. In animal models of AMI and hypertensive CM, GLP-1 infusion reduced adverse LV remodeling, improved cardiac function, and prolonged survival 117, 118. Although the data in humans is less well-established, increased activation of the GLP-1 axis leads modest weight loss, an improved lipid profile, and lower blood pressures. Moreover, in a small/non-randomized study GLP-1 infusion was associated with a significant improvement in ejection fraction in patients who presented with AMI and reduced LV function 119. Currently there are several ongoing clinical trials designed to address the impact of enhancing GLP-1 signaling on cardiovascular outcomes in diabetes. The results of these studies will provide important information about the use of these agents for the prevention and treatment of diabetic cardiovascular complications.
5. Summary
Since the term diabetic cardiomyopathy was first coined in 1972 there has been intense interest in this disease entity. With the growing population of diabetic patients the prevalence of diabetic cardiovascular disease will continue to rise. Although animal model and human studies have elucidated several pathologic features of diabetic CM, our understanding of the inciting events that lead to contractile dysfunction and/or increase myocardial susceptibility to injury remains murky. Moreover, diabetic CM as a cause of clinical HF likely involves the intersection between diabetic-myocardial reprogramming and other cardiac stressors. Given the lack of consensus on how to define or diagnose diabetic CM, there has been limited progress on developing specific treatments for this form of HF. In spite of this, mitochondrial dysfunction may explain several of the diabetic CM hallmarks such as metabolic substrate dysregulation, excess ROS generation, inflammation, and ATP depletion. Thus, targeting mitochondrial biology may lead to important new approaches to improve cardiac function in diabetics.
The currently available pharmacologic options for treating diabetes have not been studied rigorously with regards to prevention and/or treatment of diabetic CM. As a consequence, no specific recommendations can be made for the use of these agents with respect to heart failure. However, both metformin and the incretin-modulator therapies improve metabolic parameters and may be cardioprotective. In contrast, TZDs can exacerbate fluid retention making these drugs problematic in HF patients. Insulin replacement therapy should be reserved for those unresponsive to oral therapy as it may be associated with an increased risk of HF incidence and mortality. Further research of these agents in patients with early and late manifestations of diabetic CM will be important to define the optimal regimen.
Key Points.
Diabetes is associated with an increased risk of developing heart failure and portends a worse prognosis in heart failure patients.
The pathophysiology of diabetic cardiomyopathy is multifactorial, but mitochondrial dysfunction appears to be the final common pathway leading to heart failure.
The diabetic myocardium is more susceptible to injury induced by myocardial ischemia or pressure overload, leading to a further increase in heart failure among diabetics with other cardiac risk factors.
Current pharmacologic therapies for diabetes improve hyperglycemia, but the benefit in heart failure is unknown. Metformin and incretin-modulating drugs show promise as cardioprotective anti-diabetic agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease., The Framingham study. Jama. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 3.From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–9. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–67. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22:1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 7.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008;21:1144–51. doi: 10.1038/ajh.2008.252. [DOI] [PubMed] [Google Scholar]
- 9.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–9. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 10.Iliadis F, Kadoglou N, Didangelos T. Insulin and the heart. Diabetes Res Clin Pract. 2011;93 (Suppl 1):S86–91. doi: 10.1016/S0168-8227(11)70019-5. [DOI] [PubMed] [Google Scholar]
- 11.Brooks BA, Franjic B, Ban CR, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10:739–46. doi: 10.1111/j.1463-1326.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 12.Shivalkar B, Dhondt D, Goovaerts I, et al. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol. 2006;97:77–82. doi: 10.1016/j.amjcard.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 13.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail. 2011;4:53–8. doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CH, Kurup S, Herrero P, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring) 2011;19:1804–12. doi: 10.1038/oby.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–54. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond) 2011;121:43–55. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu MS, Liang JT, Lin YD, Wu ET, Tseng YZ, Chang KC. Aminoguanidine prevents the impairment of cardiac pumping mechanics in rats with streptozotocin and nicotinamide-induced type 2 diabetes. Br J Pharmacol. 2008;154:758–64. doi: 10.1038/bjp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wold LE, Ceylan-Isik AF, Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin. 2005;26:908–17. doi: 10.1111/j.1745-7254.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–55. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrero P, Peterson LR, McGill JB, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh W, Abel ED, Breslow JL, et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–27. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 29.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:219–26. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 30.Gray S, Kim JK. New insights into insulin resistance in the diabetic heart. Trends Endocrinol Metab. 2011;22:394–403. doi: 10.1016/j.tem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan JG. Peroxisome proliferator activated receptor-alpha (PPARalpha) and PPAR gamma coactivator-1alpha (PGC-1alpha) regulation of cardiac metabolism in diabetes. Pediatr Cardiol. 2011;32:323–8. doi: 10.1007/s00246-011-9889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–45. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 34.Wu P, Peters JM, Harris RA. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem Biophys Res Commun. 2001;287:391–6. doi: 10.1006/bbrc.2001.5608. [DOI] [PubMed] [Google Scholar]
- 35.Wright JJ, Kim J, Buchanan J, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–60. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 37.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 38.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–5. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 39.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–9. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 40.van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10–8. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 41.Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–33. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 43.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–26. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park TS, Yamashita H, Blaner WS, Goldberg IJ. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol. 2007;18:277–82. doi: 10.1097/MOL.0b013e32814a57db. [DOI] [PubMed] [Google Scholar]
- 45.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–7. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Chambers KT, Leone TC, Sambandam N, et al. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–62. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr Diabetes Rev. 2009;5:120–44. doi: 10.2174/157339909788166800. [DOI] [PubMed] [Google Scholar]
- 48.Serpillon S, Floyd BC, Gupte RS, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–62. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aliciguzel Y, Ozen I, Aslan M, Karayalcin U. Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J Lab Clin Med. 2003;142:172–7. doi: 10.1016/S0022-2143(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 50.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 51.Ye G, Metreveli NS, Donthi RV, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 52.Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–83. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- 53.Matsushima S, Kinugawa S, Ide T, et al. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am J Physiol Heart Circ Physiol. 2006;291:H2237–45. doi: 10.1152/ajpheart.00427.2006. [DOI] [PubMed] [Google Scholar]
- 54.Choi KM, Zhong Y, Hoit BD, et al. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- 55.Pereira L, Matthes J, Schuster I, et al. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–15. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 56.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 57.Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:715–24. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe K, Thandavarayan RA, Harima M, et al. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Curr Cardiol Rev. 2010;6:280–90. doi: 10.2174/157340310793566145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Wang J, Takeda N, Binaglia L, Panagia V, Dhalla NS. Changes in cardiac protein kinase C activities and isozymes in streptozotocin-induced diabetes. Am J Physiol. 1999;277:E798–804. doi: 10.1152/ajpendo.1999.277.5.E798. [DOI] [PubMed] [Google Scholar]
- 60.Wieland T, Lutz S, Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr Opin Pharmacol. 2007;7:201–7. doi: 10.1016/j.coph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Way KJ, Isshiki K, Suzuma K, et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51:2709–18. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Lei S, Gao X, et al. PKCbeta inhibition with ruboxistaurin reduces oxidative stress and attenuates left ventricular hypertrophy and dysfunction in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 2012;122:161–73. doi: 10.1042/CS20110176. [DOI] [PubMed] [Google Scholar]
- 63.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 64.Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–7. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 65.Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–46. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westermann D, Van Linthout S, Dhayat S, et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes. 2007;56:1834–41. doi: 10.2337/db06-1662. [DOI] [PubMed] [Google Scholar]
- 67.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 68.Chowdhry MF, Vohra HA, Galinanes M. Diabetes increases apoptosis and necrosis in both ischemic and nonischemic human myocardium: role of caspases and poly-adenosine diphosphate-ribose polymerase. J Thorac Cardiovasc Surg. 2007;134:124–31. 31, e1–3. doi: 10.1016/j.jtcvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 69.Barouch LA, Gao D, Chen L, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–24. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 70.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3:219–28. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu M, Umeda K, Sugihara N, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–6. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regan TJ, Lyons MM, Ahmed SS, et al. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977;60:884–99. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van den Bergh A, Vanderper A, Vangheluwe P, et al. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–9. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 74.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575–96. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Linthout S, Seeland U, Riad A, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–27. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 76.Li Q, Sun SZ, Wang Y, Tian YJ, Liu MH. The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in the hearts of STZ-induced diabetic rats. Acta Cardiol. 2007;62:485–91. doi: 10.2143/AC.62.5.2023412. [DOI] [PubMed] [Google Scholar]
- 77.Bollano E, Omerovic E, Svensson H, Waagstein F, Fu M. Cardiac remodeling rather than disturbed myocardial energy metabolism is associated with cardiac dysfunction in diabetic rats. Int J Cardiol. 2007;114:195–201. doi: 10.1016/j.ijcard.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 78.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitra R, Nogee DP, Zechner JF, et al. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–66. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 81.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 82.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 83.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–8. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniels A, Linz D, van Bilsen M, et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail. 2012;14:193–201. doi: 10.1093/eurjhf/hfr166. [DOI] [PubMed] [Google Scholar]
- 85.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–41. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 86.Cosyns B, Droogmans S, Weytjens C, et al. Effect of streptozotocin-induced diabetes on left ventricular function in adult rats: an in vivo Pinhole Gated SPECT study. Cardiovasc Diabetol. 2007;6:30. doi: 10.1186/1475-2840-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basu R, Oudit GY, Wang X, et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297:H2096–108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 88.Wold LE, Ren J. Streptozotocin directly impairs cardiac contractile function in isolated ventricular myocytes via a p38 map kinase-dependent oxidative stress mechanism. Biochem Biophys Res Commun. 2004;318:1066–71. doi: 10.1016/j.bbrc.2004.04.138. [DOI] [PubMed] [Google Scholar]
- 89.Nathan DM, Meigs J, Singer DE. The epidemiology of cardiovascular disease in type 2 diabetes mellitus: how sweet it is … or is it? Lancet. 1997;350(Suppl 1):SI4–9. doi: 10.1016/s0140-6736(97)90021-0. [DOI] [PubMed] [Google Scholar]
- 90.Savage MP, Krolewski AS, Kenien GG, Lebeis MP, Christlieb AR, Lewis SM. Acute myocardial infarction in diabetes mellitus and significance of congestive heart failure as a prognostic factor. Am J Cardiol. 1988;62:665–9. doi: 10.1016/0002-9149(88)91199-x. [DOI] [PubMed] [Google Scholar]
- 91.Rytter L, Troelsen S, Beck-Nielsen H. Prevalence and mortality of acute myocardial infarction in patients with diabetes. Diabetes Care. 1985;8:230–4. doi: 10.2337/diacare.8.3.230. [DOI] [PubMed] [Google Scholar]
- 92.Czyzk A, Krolewski AS, Szablowska S, Alot A, Kopczynski J. Clinical course of myocardial infarction among diabetic patients. Diabetes Care. 1980;3:526–9. doi: 10.2337/diacare.3.4.526. [DOI] [PubMed] [Google Scholar]
- 93.Kouvaras G, Cokkinos D, Spyropoulou M. Increased mortality of diabetics after acute myocardial infarction attributed to diffusely impaired left ventricular performance as assessed by echocardiography. Jpn Heart J. 1988;29:1–9. doi: 10.1536/ihj.29.1. [DOI] [PubMed] [Google Scholar]
- 94.Yudkin JS, Oswald GA. Determinants of hospital admission and case fatality in diabetic patients with myocardial infarction. Diabetes Care. 1988;11:351–8. doi: 10.2337/diacare.11.4.351. [DOI] [PubMed] [Google Scholar]
- 95.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]
- 96.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–53. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 97.Thakker GD, Frangogiannis NG, Bujak M, et al. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2504–14. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 98.Shiomi T, Tsutsui H, Ikeuchi M, et al. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol. 2003;42:165–72. doi: 10.1016/s0735-1097(03)00509-6. [DOI] [PubMed] [Google Scholar]
- 99.Cittadini A, Mantzoros CS, Hampton TG, et al. Cardiovascular abnormalities in transgenic mice with reduced brown fat: an animal model of human obesity. Circulation. 1999;100:2177–83. doi: 10.1161/01.cir.100.21.2177. [DOI] [PubMed] [Google Scholar]
- 100.Estep JD, Aguilar D. Diabetes and heart failure in the post-myocardial infarction patient. Curr Heart Fail Rep. 2006;3:164–9. doi: 10.1007/s11897-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 101.Lindman BR, Arnold SV, Madrazo JA, et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–92. doi: 10.1161/CIRCHEARTFAILURE.110.960039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falcao-Pires I, Hamdani N, Borbely A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–9. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 103.Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–7. doi: 10.1016/j.amjcard.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail. 2012;14:176–84. doi: 10.1093/eurjhf/hfr170. [DOI] [PubMed] [Google Scholar]
- 105.Shi C, Wang LJ, Hu DF, et al. Prevalence, clinical characteristics and outcome in patients with chronic heart failure and diabetes. Chin Med J (Engl) 2010;123:646–50. [PubMed] [Google Scholar]
- 106.Eguchi K, Kario K, Hoshide S, Ishikawa J, Morinari M, Shimada K. Type 2 diabetes is associated with left ventricular concentric remodeling in hypertensive patients. Am J Hypertens. 2005;18:23–9. doi: 10.1016/j.amjhyper.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 107.Nichols GA, Koro CE, Gullion CM, Ephross SA, Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev. 2005;21:51–7. doi: 10.1002/dmrr.480. [DOI] [PubMed] [Google Scholar]
- 108.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J. 2005;149:168–74. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 109.Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ. 2007;335:508–12. doi: 10.1136/bmj.39255.669444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. Bmj. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero SP, Andrey JL, Garcia-Egido A, et al. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.10.141. [DOI] [PubMed] [Google Scholar]
- 112.Pop-Busui R, Lombardero M, Lavis V, et al. Relation of severe coronary artery narrowing to insulin or thiazolidinedione use in patients with type 2 diabetes mellitus (from the Bypass Angioplasty Revascularization Investigation 2 Diabetes Study) Am J Cardiol. 2009;104:52–8. doi: 10.1016/j.amjcard.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Erdmann E, Charbonnel B, Wilcox R. Thiazolidinediones and cardiovascular risk - a question of balance. Curr Cardiol Rev. 2009;5:155–65. doi: 10.2174/157340309788970333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30:2773–8. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 115.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–90. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 116.Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–76. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 117.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 118.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–60. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]