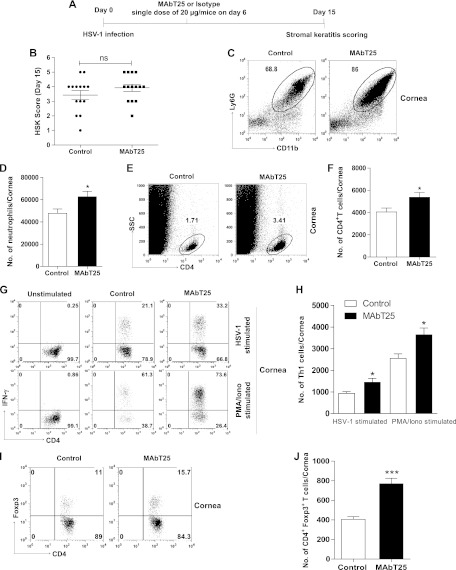
Fig 4.
MAbT25 administration during early clinical phase of disease was not effective in controlling the severity of SK lesions. (A) C57BL/6 mice infected with 1 × 104 PFU HSV-1 were treated with MAbT25 or isotype antibody on day 6 p.i. (B) SK lesion scores of MAbT25-treated and control mice on day 15 p.i. Experiments were repeated three times with 8 to 10 mice per group. One-way ANOVA with Tukey's multiple-comparison test was used to calculate significance (P value not significant, P > 0.05). (C to F) HSV-infected corneas were pooled and processed as previously described for FACS analysis. (C) FACS plots showing neutrophils in the corneas of control and MAbT25-treated groups; (D) bar graphs showing neutrophil numbers; (E) FACS plots showing the total CD4+ T cells in the corneas; (F) bar graphs showing CD4+ T cell numbers. (G) Representative FACS plots showing CD4+ IFN-γ+ cells. (Top) IFN-γ+ cells on HSV-1 stimulation; (bottom) PMA-ionomycin stimulation in MAbT25-treated and control groups. (H) Bar graphs representing CD4+ IFN-γ+ T cell numbers. (I) FACS plots showing Foxp3+ Tregs in the corneas of control and MAbT25-treated groups. (J) Bar graphs representing numbers of Foxp3+T cells. Data represent means ± SEMs of 6 to 8 mice per group from at least two independent experiments. Statistical levels of significance were calculated by Student's t test (*, P < 0.05; ***, P < 0.0001).