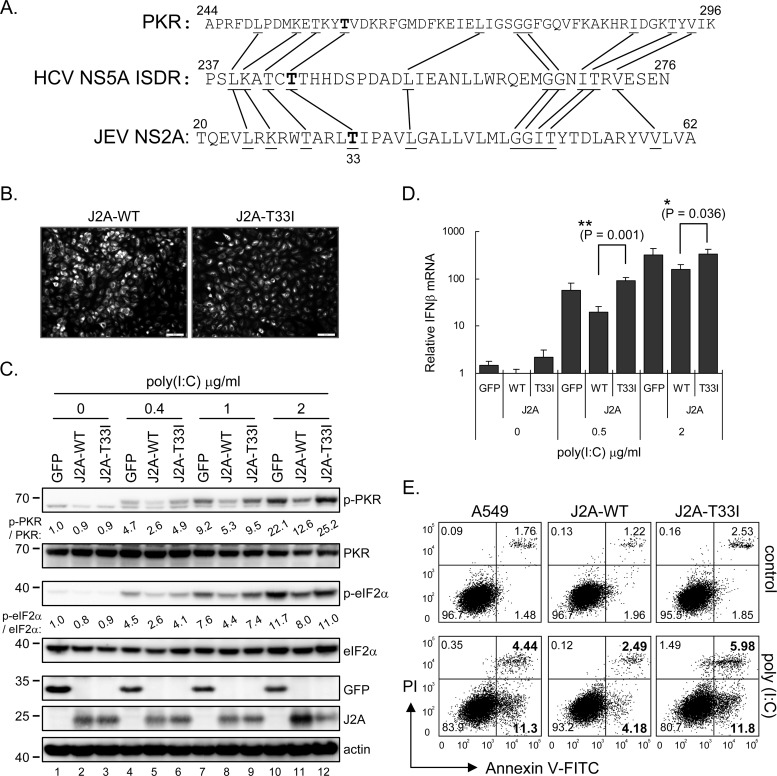
Fig 7.
JNS2A-mediated PKR blockage was attenuated by T33I substitution. (A) JNS2A amino acid sequences (residues 20 to 62) were compared to that of PKR dimerization domain (residues 244 to 296) and HCV NS5A ISDR (interferon sensitivity-determining region) (residues 237 to 276). Identical amino acids among these three proteins and the Thr-33 of JNS2A are highlighted by lines and boldfacing, respectively. (B) A JNS2A T33I mutation, a Thr-to-Ile change of NS2A residue 33, was generated by single primer mutation strategy, and stable A549 cells with NS2A-T33I expression were established by lentivirus transduction. The expression of NS2A wild-type (J2A-WT) and T33I mutant (J2A-T33I) in stable cell lines were determined by IFA with anti-HA antibody. (C) Stable A549 cells with HA-tagged GFP, J2A-WT, and J2A-T33I were treated with the indicated doses of poly(I·C) (0, 0.4, 1, and 2 μg/ml) for 1 h and harvested for Western blotting with the indicated antibodies. (D) dsRNA-induced type Ι IFN production in J2A-WT, J2A-T33I, and GFP control cells was detected by RT-qPCR after poly(I·C) stimulation for 6 h. The relative IFN-β mRNA level was normalized to that of actin (n = 3). *, P < 0.05; **, P < 0.005 (two-tailed Student t test). (E) Flow cytometry of cells stimulated with 2 μg of poly(I·C)/ml for 8 h and then stained with annexin V-FITC and propidium iodide (PI; Biovision). The proportions of cells in each quadrant are shown.