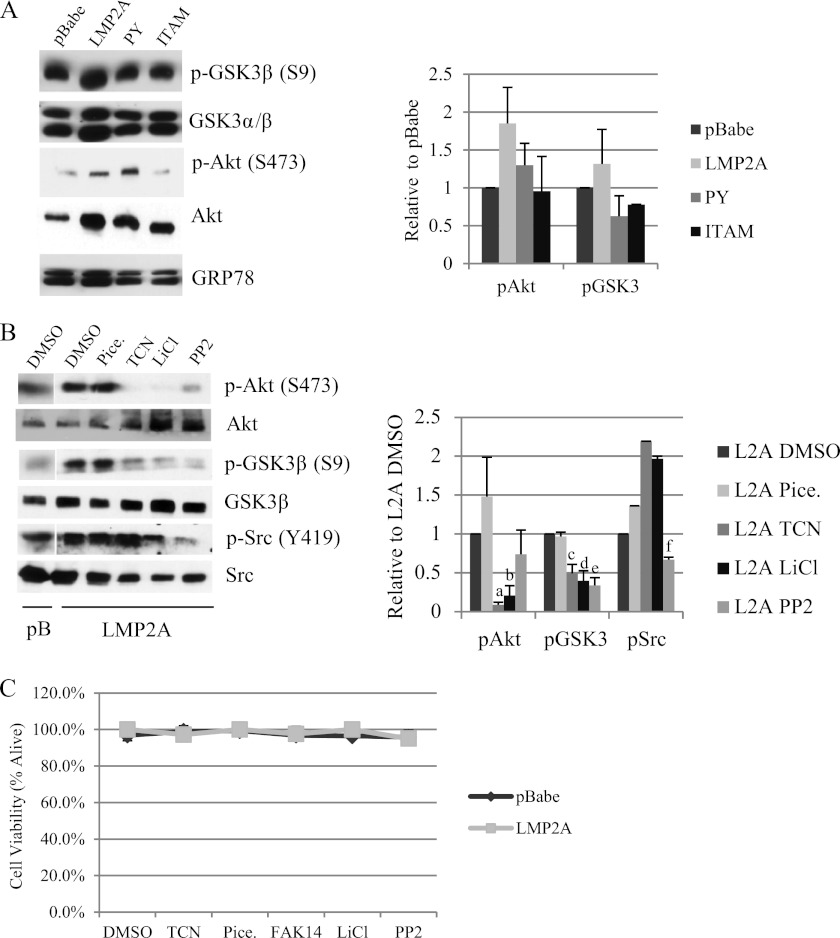
Fig 4.
LMP2A induces Akt signaling and activation of focal adhesion kinase. (A) Protein expression of Wnt signaling mediators was measured by Western blotting in cytoplasmic lysates from HaCaT cells expressing pBabe, wild-type LMP2A, and the LMP2A PY and ITAM mutants. Equal loading was determined by expression of the endoplasmic reticulum protein GRP78. Expression of phosphoproteins was normalized to total levels and is expressed relative to LMP2A DMSO vehicle controls; averages from three experiments are presented. (B) Protein levels of Akt, GSK3β, and Src were determined by Western blotting using antibodies to total protein or phosphorylated residues. Whole-cell lysates from pBabe and wild-type LMP2A expressing HaCaT cells were prepared from cells following treatment with DMSO vehicle control, the Syk inhibitor piceatannol (25 μM), the Akt inhibitor triciribine (TCN; 5 μM), the GSK3 inhibitor LiCl (10 mM), and the Src inhibitor PP2 (500 nM). Expression of phosphoprotein was normalized to total levels and expressed relative to values for LMP2A DMSO vehicle controls, and quantitation of two experiments is presented. a, P = 4.42 × 10−9 versus LMP2A DMSO; b, P = 0.00027 versus LMP2A DMSO; c, P = 0.0272 versus LMP2A DMSO; d, P = 0.0267 versus LMP2A DMSO; e, P = 0.011 versus LMP2A DMSO; f, P = 0.00587 versus LMP2A DMSO. (C) HaCaT cells expressing pBabe and wild-type LMP2A were treated overnight with DMSO vehicle control, triciribine (5 μM), piceatannol (25 μM), FAK14 (1 μM), LiCl (10 mM), and PP2 (500 nM). Cells were collected 24 h after treatment and cell viability was determined by trypan blue exclusion.