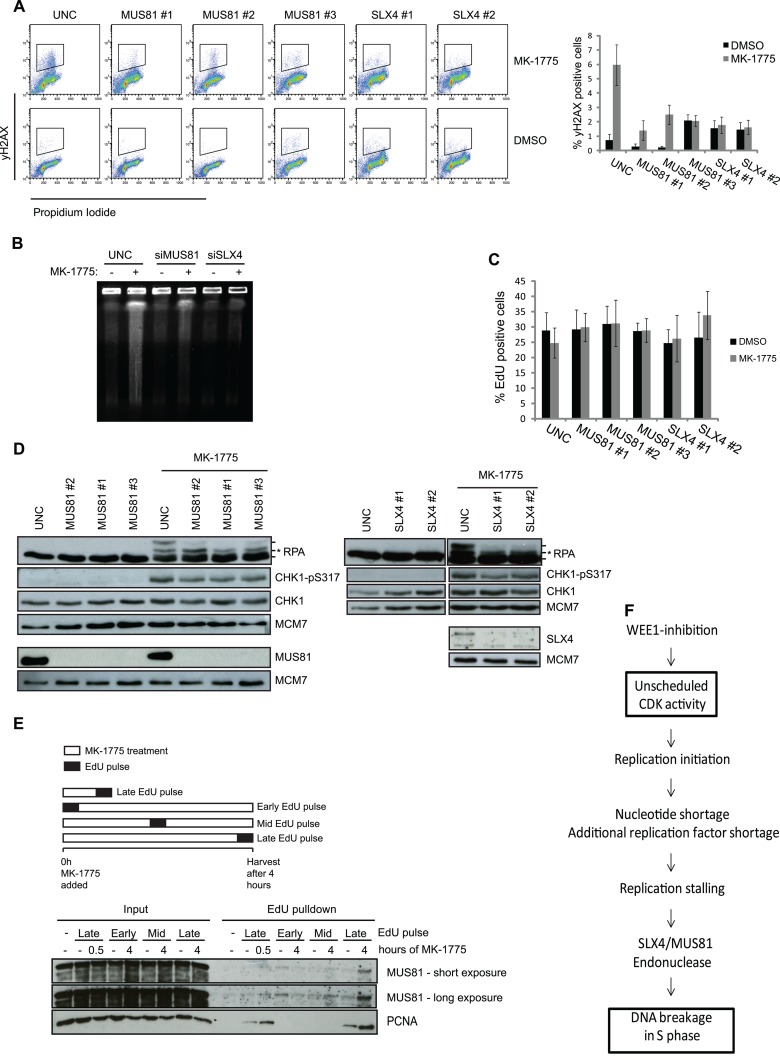
Fig 5.
DNA DSBs formation but not ATR signaling is mediated by SLX4-MUS81 after WEE1 inhibition. (A) U2OS cells were transfected with siRNAs targeting MUS81 and SLX4. Transfection with UNC was included as a negative control. The cells were treated with EdU (10 μM) for 30 min prior to harvest, and at 48 h posttransfection the cells were harvested and processed for FACS. Cells were stained with PI and antibody against γH2AX, and EdU was detected through the Click-iT reaction. FACS analysis was carried out with cells stained for γH2AX and PI, and data are means ± SEM from a minimum of five experiments. (B) U2OS cells were reverse transfected with siRNA targeting MUS81 and SLX4 for 48 h, treated with the WEE1 inhibitor MK-1775 (2 μM) for 4 h, and processed for PFGE. (C) EdU graphs from the experiment whose results are shown in panel A. (D) Cells were treated as for panel A and then harvested and processed for immunoblotting. Cell lysates were stained with antibodies against RPA, CHK1-p317, CHK1, MUS81, SLX4, and MCM7 as a loading control. In the RPA panels, the asterisk shows the ATR-dependent RPA phosphorylation, whereas the upper RPA band is the DNA-PK mediated phosphorylation of S4 and S8. (E) Cells were pulsed with 12 min of EdU at the start, middle, or end of MK-1775 treatment. The cells were harvested at the indicated time points and processed for iPOND to pull down nascent proteins. Lysates were processed for immunoblotting using antibodies against MUS81 and PCNA. A minus sign denotes a non-Click-iT control, where DMSO was used instead of biotin-azide to detect incorporated EdU. (F) Diagram depicting how increased CDK activity following WEE1 inhibition can lead to DNA breakage in S-phase cells via increased origin firing and ensuing enhanced nucleotide consumption.