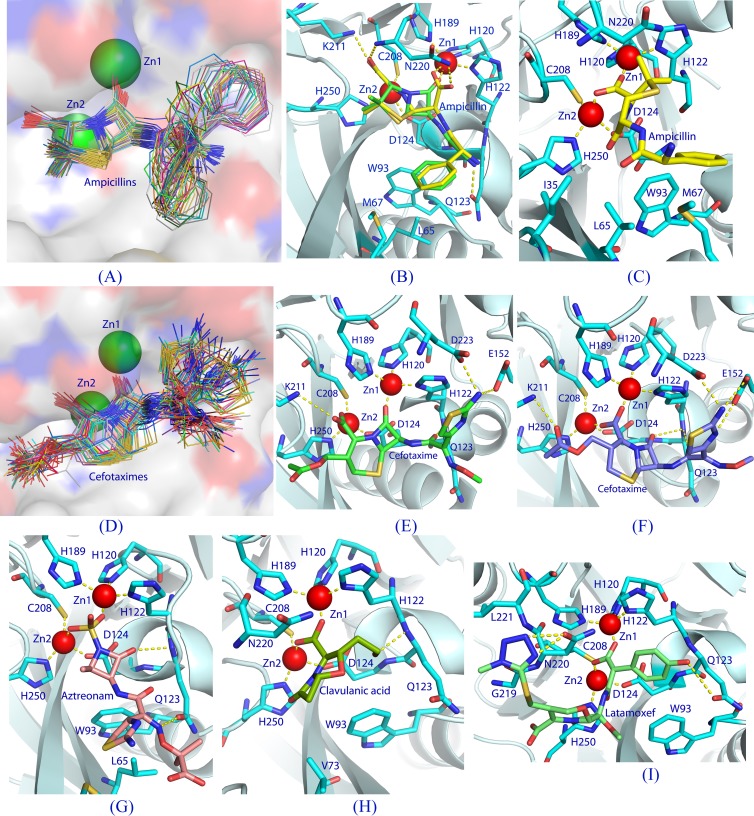
Fig 4.
Docking of β-lactams into the NDM-1 binding pocket. (A) A plot of all S conformers of docked ampicillin (total, 89 sticks) into the NDM-1 active site (surface presentation). Positional variation of the phenyl groups forms a pattern like the open tail of a peacock. (B) Interactions between NDM-1 and the S conformer (green sticks) of ampicillin with the lowest binding energy. The dotted lines represent hydrogen bonds or coordination with the metals. The hydrolyzed ampicillin from the crystal structure of the NDM-1-product complex (PDB accession number 3Q6X) is plotted with yellow sticks. (C) Interactions of the I conformer of ampicillin with NDM-1. (D) Binding of all the S conformers (71 total) of cefotaxime to the NDM-1 pocket. (E) Interactions of the S conformer of cefotaxime with NDM-1. (F) Interactions of the I conformer of cefotaxime with NDM-1. (G) An inhibitory conformation of aztreonam. (H) A representative binding of clavulanic acid. (I) An inhibitory binding of moxalactam (latamoxef) to NDM-1 in which the tyrosyl-carboxylic group of moxalactam interacts with both zinc ions.