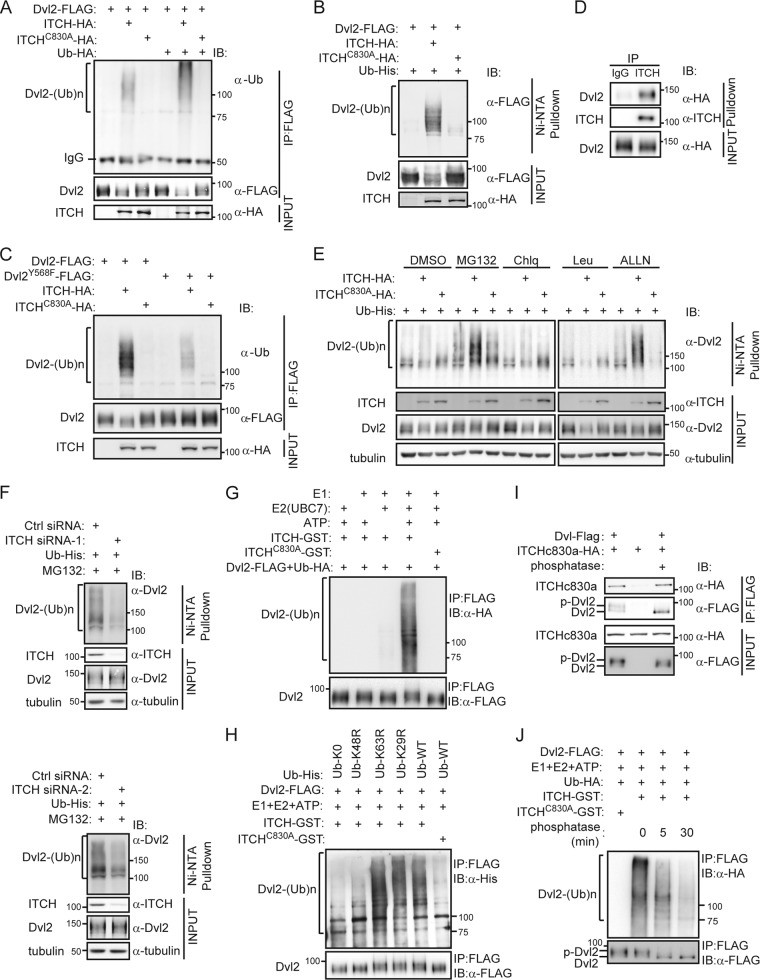
Fig 3.
ITCH ubiquitinates and promotes degradation of phosphorylated Dvl in a proteasome-dependent way. (A and B) ITCH was found to ubiquitinate exogenous Dvl2. HEK293T cells were transfected with the indicated plasmids. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody, and ubiquitin conjugates were then detected using antiubiquitin antibody (A) or subjected to Ni2+-agarose beads for pulling down His-ubiquitinated proteins; Ub-His-conjugated Dvl2-FLAG was then detected using anti-FLAG antibody (B). (C) The Dvl2-Y568F mutant showed less ubiquitination induced by ITCH than did wild-type Dvl2. The experiment was performed as described for panel A. (D) Stably expressed HA-Dvl2 could be coimmunoprecipitated by ITCH in Dvl2-293 cells. Cell extracts were immunoprecipitated with ITCH antibody. Dvl2-HA was detected by anti-HA antibody. (E) Dvl2 was ubiquitinated by ITCH and degraded in a proteasome-dependent pathway. Dvl2-293 stable cells were transfected with the indicated plasmids for 16 h and then treated with 25 μM MG132, 100 μM chloroquine (Chlq), 100 μM ALLN, or 50 μM leupeptin (Leu) separately, for an additional 5 h. His-ubiquitinated proteins were pulled down using Ni2+-agarose beads and subjected to Western blotting. DMSO, dimethyl sulfoxide. (F) Knockdown of ITCH reduced the ubiquitination of Dvl2. Dvl2-293 stable cells were transfected with Ctrl siRNA or ITCH siRNA (ITCH siRNA-1 or siRNA-2). Twenty-four hours later, Ub-His was transfected, and after another 24 h, cells were treated with 25 μM MG132 for an additional 5 h. His-ubiquitinated proteins were pulled down using Ni2+-agarose beads. (G) In vitro assay of Dvl2 ubiquitination. Using the immunoprecipitated Dvl2-FLAG from the transfected HEK293T cells as the substrate, in vitro experiments were carried out as described in Materials and Methods. (H) ITCH ubiquitinated Dvl by K48-linked chains. Ub-WT-His and several Ub mutants (Ub-K29R-His, Ub-K48R-His, Ub-K63R-His, and Ub-K0-His) were used as indicated for an in vitro ubiquitination assay. (I) Phosphorylation of Dvl is not required for its interaction with ITCH. Dvl2-FLAG and ITCH-C830A-HA were overexpressed separately in different pools of HEK293T cells, and then the Dvl2-FLAG-containing cell lysate was treated with or without alkaline phosphatase and subsequently mixed with the ITCH-C830A-HA-containing cell lysate, followed by co-IP assay. (J) ITCH prefers to ubiquitinate phosphorylated Dvl2. Immunoprecipitated Dvl2-FLAG from 293T cell lysates were treated with alkaline phosphatase for 5 or 30 min at 30°C before the in vitro ubiquitination reaction.