Fig 1.
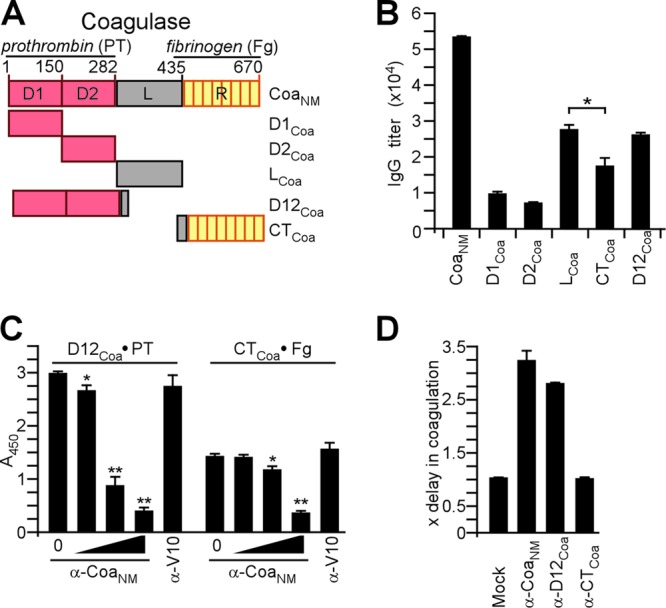
Immune responses to coagulase. (A) Drawing to illustrate the primary structure of coagulase from S. aureus Newman (CoaNM), which was purified from E. coli via an N-terminal His6 tag. CoaNM encompasses the D1 and D2 domains involved in prothrombin (PT) binding, the linker (L) domain, and the repeat (R) domain, which is comprised of tandem repeats of a 27-residue peptide sequence that binds to fibrinogen (Fg). In addition to CoaNM, the D1Coa, D2Coa, D12Coa, LCoa, and RCoa domains were purified. (B) Rabbits were immunized with purified CoaNM, and immune sera were examined by an ELISA for serum IgG reactive with CoaNM, D1Coa, D2Coa, D12Coa, LCoa, or CTCoa. Statistical analysis was performed with the Student two-tailed t test (*, P < 0.05). (C) The association of D12Coa with human prothrombin was measured by an ELISA and perturbed with increasing concentrations of rabbit IgG directed against CoaNM (0 μM, 0.012 μM, 0.12 μM, or 1.2 μM) or the plague vaccine antigen V10 (1.2 μM) as a control. The association of CTCoa with fibrinogen was measured by an ELISA and perturbed with increasing concentrations of rabbit IgG directed against CoaNM (0 μM, 0.017 μM, 0.17 μM, or 1.7 μM) or the plague vaccine antigen V10 (1.7 μM) as a control. (D) Affinity-purified rabbit IgG specific for CoaNM (α-CoaNM), D12Coa (α-D12Coa), or CTCoa (α-CTCoa) was added to citrate-treated mouse blood and inoculated with S. aureus Newman to monitor the inhibition of staphylococcal coagulation.
