Figure 3. Wild-type full-length Fus1 down regulates c-Abl tyrosine kinase.
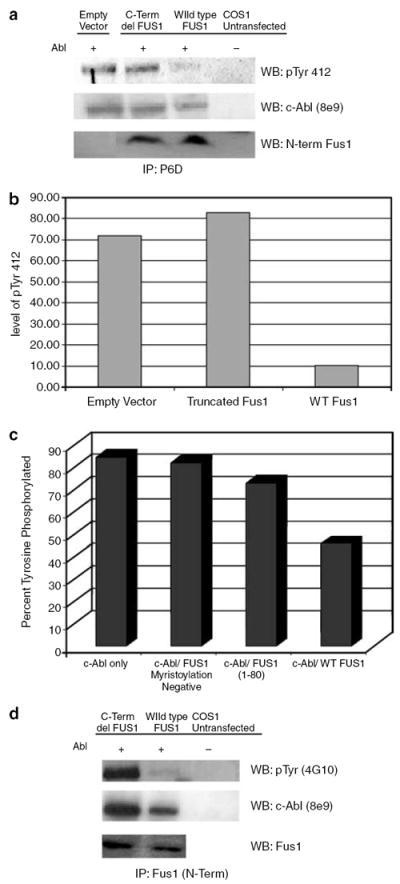
(a) c-ABL was co-transfected with either wild-type full-length FUS1, truncated FUS1, or empty vector in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and lysate was incubated with the monoclonal anti-Abl antibody P6D. The immune complex was pulled down using protein A beads and subjected to western blotting. Antibody specific for pTyr412 of Abl (Abcam) was used to show kinase active c-Abl. The same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9. N-terminal Fus1 antibody was used to detect Fus1. (b) The intensities of the c-Abl pTyr 412 bands were normalized against c-Abl expression. (c) C-ABL was co-transfected with either wild-type full-length FUS1, truncated FUS1 or a non-myristoylated mutant FUS1 (Gly2Ala) in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and the lysate was incubated with the monoclonal anti-Abl antibody P6D. The immune complex was pulled down using protein A beads and subjected to western blotting. Phosphotyrosine c-Abl was detected by anti-phosphotyrosine antibody 4G10 and the same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9 . The band intensities were normalized for c-Abl expression. (d) C-ABL was co-transfected with either wild-type full-length FUS1 or truncated FUS1 in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and lysate was incubated with a polyclonal N-terminal Fus1 antibody. The immune complex was pulled down using protein A beads and subjected to western blotting. Phosphotyrosine c-Abl was detected by anti-phosphotyrosine antibody 4G10 and the same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9.
