Table 1.
Reaction optimisation.a
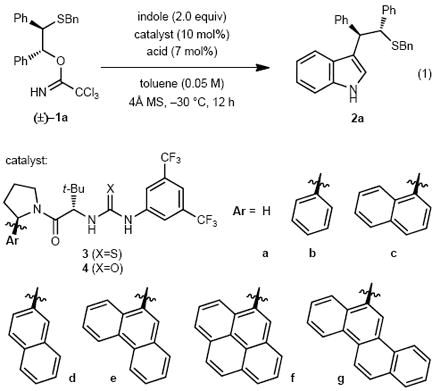
| ||||
|---|---|---|---|---|
| entry | catalyst | acid | yield (%)b | ee (%)c |
| 1 | 3b | HCl | 10 | 5 |
| 2 | 3b | HOTf | 73 | 32 |
| 3 | 3b | FSO3H | 78 | 19 |
| 4 | 3b | 2,4-diNBSA | 79 | 63 |
| 5 | 3b | 4-NBSA | 72 | 73 |
| 6 | – | 4-NBSA | 7 | n/a |
| 7 | 3a | 4-NBSA | 16 | 12 |
| 8 | 3c | 4-NBSA | 84 | 84 |
| 9 | 3d | 4-NBSA | 80 | 85 |
| 10 | 3e | 4-NBSA | 93 | 93 |
| 11 | 3f | 4-NBSA | 91 | 91 |
| 12 | 3g | 4-NBSA | 97 | 88 |
| 13 | 4e | 4-NBSA | 98 | 92 |
Optimisation reactions were performed on 0.05 mmol scale.
Isolated yields of material purified chromatographically.
Enantiomeric excesses (ee’s) determined by HPLC analysis.
MS = molecular sieves; 2,4-diNBSA = 2,4-dinitrobenzenesulfonic acid; 4-NBSA = 4-nitrobenzenesulfonic acid.
