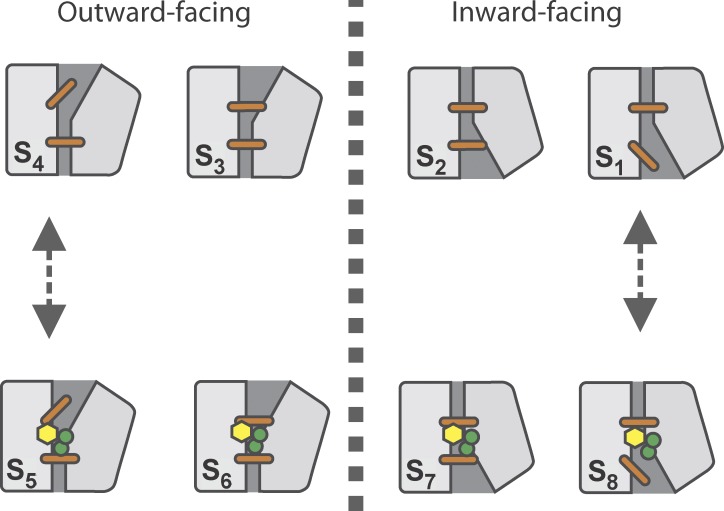
Figure 1.
Cotransport mechanism of SGLT1 suggested by the structural data of the LeuT fold family. In state S1, the cotransporter is in its inward open conformation. The transition from S1 to S2 closes the intracellular thin gate (orange stick), thus occluding the binding sites. A major conformational change (S2→S3) then reorients SGLT1 to its outward facing state. The subsequent opening of the extracellular thin gate (S3→S4) makes it possible for SGLT1 to bind Na+ ions (green circles) and glucose (yellow hexagon; S4→S5). The extracellular thin gate is then free to close (S5→S6), which provokes a structural rearrangement of the fully loaded transporter leading to the intracellular facing occluded state S7. This is followed by the opening of the intracellular thin gate (S7→S8), allowing intracellular release of the substrates (S8→S1).