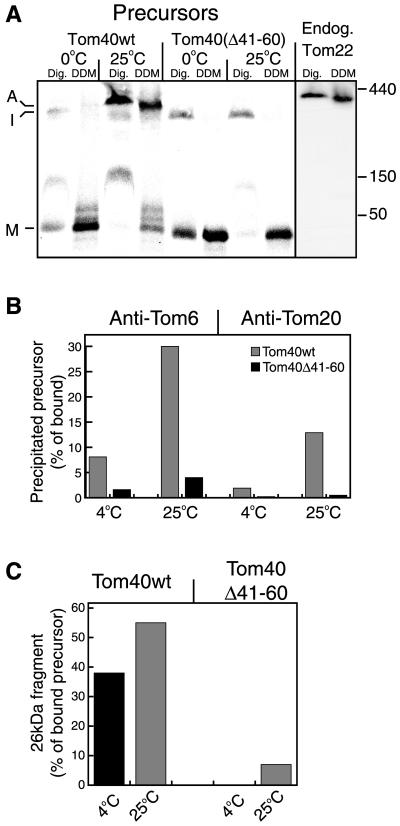
Figure 2.
Tom40 variant lacking amino acid residues 41–60 is not integrated into the TOM complex. (A) Radiolabeled precursors were incubated at 0°C or 25°C with 50 μg mitochondria for 20 min. All samples were reisolated and solubilized in buffer containing either 1% digitonin (Dig.) or 0.3% dodecylmaltoside (DDM). After a clarifying spin, the solubilized mitochondria were loaded on a blue native gel. For detection of the endogenous TOM complex, antibodies against Tom22 were used. The three main stages of import are indicated: M, bound monomer; I, high-molecular-weight intermediate; and A, assembled TOM core complex. The positions of molecular weight markers (kDa) are indicated on the right side of A. (B) Tom40Δ41–60 is not stably associated with Tom20 and Tom6. Tom40 (Tom40wt) and Tom40Δ41–60 radiolabeled precursors were incubated with mitochondria for 20 min at 4°C or 25°C and mitochondria were reisolated. One aliquot from each import reaction was directly analyzed by SDS-PAGE and was taken as total bound precursor. A second aliquot was solubilized with 0.5% digitonin and split into three aliquots that were subjected to immunoprecipitation with antibodies against either Tom6, Tom20, or with antibodies from preimmune serum. The latter precipitated only background levels of the precursors (our unpublished results). The lower amounts of assembled precursor observed with Tom20 antibodies are likely due to the fact that Tom20 is only loosely bound to the TOM complex (Ahting et al., 1999). (C) Tom40 can acquire its correctly folded state despite lack of integration into the TOM complex. A third aliquot of the import reactions described in B was treated with proteinase K, and the formation of the characteristic 26-kDa fragment was quantified.