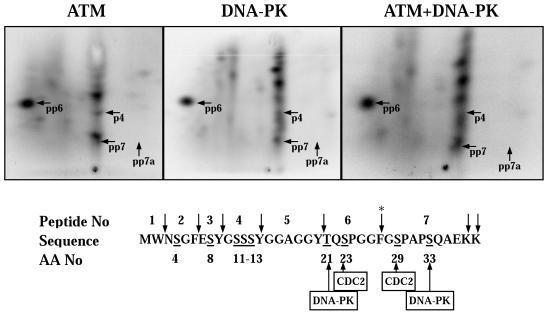
Figure 4.
Chymotryptic/tryptic phosphopeptide maps of in vitro–phosphorylated recombinant RPA-p34. (Top) Five hundred nanograms of purified recombinant RPA complex was phosphorylated by either purified ATM or 10 U of DNA-PKcs/Ku for 30 min. Hyperphosphorylated RPA-p34 was separated by SDS-PAGE and transferred to a PVDF membrane. The hyperphosphorylated form of RPA-p34 was detected by phosphorimager analysis and excised from the membrane. The hyperphosphorylated RPA-p34 was digested twice with 10 μg of chymotrypsin/trypsin and oxidized with performic acid. The digested peptides were loaded onto TLC plates and separated by electrophoresis at pH 1.9 in the first dimension, followed by ascending chromatography in the second dimension. The labeled peptides were detected by phosphorimager analysis. To verify identical and unique peptides, equal ra-dioactivity from digests of DNA-PKcs/Ku and ATM-phosphorylated RPA-p34 were loaded onto the same chromatography plate and subjected to two-dimensional separation. Numbered tryptic/chymotryptic peptides indicated on the maps with arrows refer to the peptide sequence number (lower panel) with a letter “p” designating a phosphorylated serine or threonine on the peptide. (bottom) The amino acid sequence of the N-terminus of RPA-p34 is shown along with the sites for cleavage by trypsin/chymotrypsin (↓), sites phosphorylated by Cdc2p34/cyclin B and consensus sites for DNA-PKcs/Ku. The asterisk denotes the cleavage site that is blocked by adjacent phosphorylated amino acids.