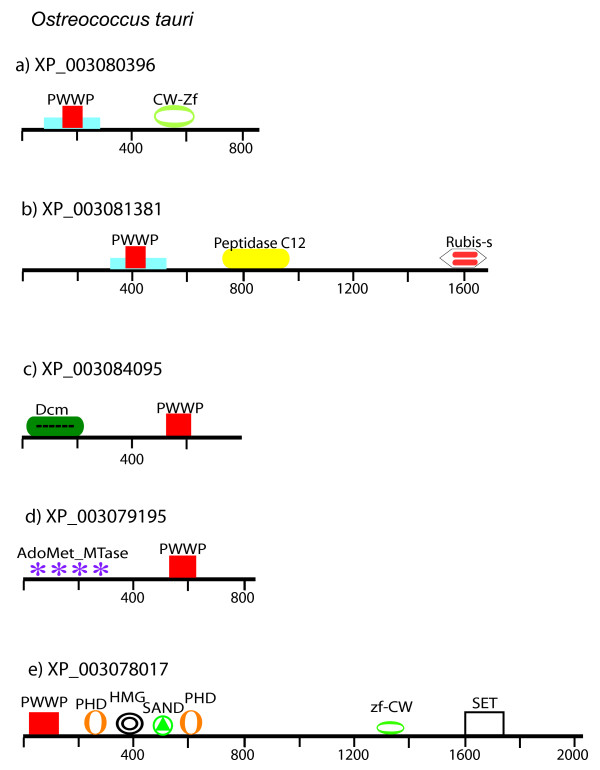
Figure 2 .
Domain architecture of the PWWP-containing proteins in Ostreococcus tauri. The five PWWP-containing proteins are drawn to scale. a) The PWWP domain (red box) is within a region defined as a Tudor/PWWP/MBT superfamily domain (region in blue) in combination with a domain related to a CW-type zinc finger. Note that this structure is related also to the Set domain fold. b) an O. tauri specific protein with a similarly organized PWWP domain in a unique combination with an Ubiquitin carboxyl-terminal hydrolase family 1 domain (most similar to ubiquitinases of animal origin) and with a domain from the RuBisCo LSMT substrate-binding protein. The latter domain shows the highest similarity to RuBisCo holoenzyme complex proteins from other algae, but not to the Chlamydomonas, Volvox, or the green plants. This domain is also related to the SET domain carrying a histone methyltransferase activity; c) a putative DNA-methyltransferase most similar to proteins from other ocean metagenomes; d) a putative DNA-methyltransferase highly similar to the bacterial methyltransferases; e) an architecturally complex protein related to the proteins of land plants grouped in the ATX3/4/5 clade but the HMG, the SAND, and the zf-CW domains are absent from the plant versions.