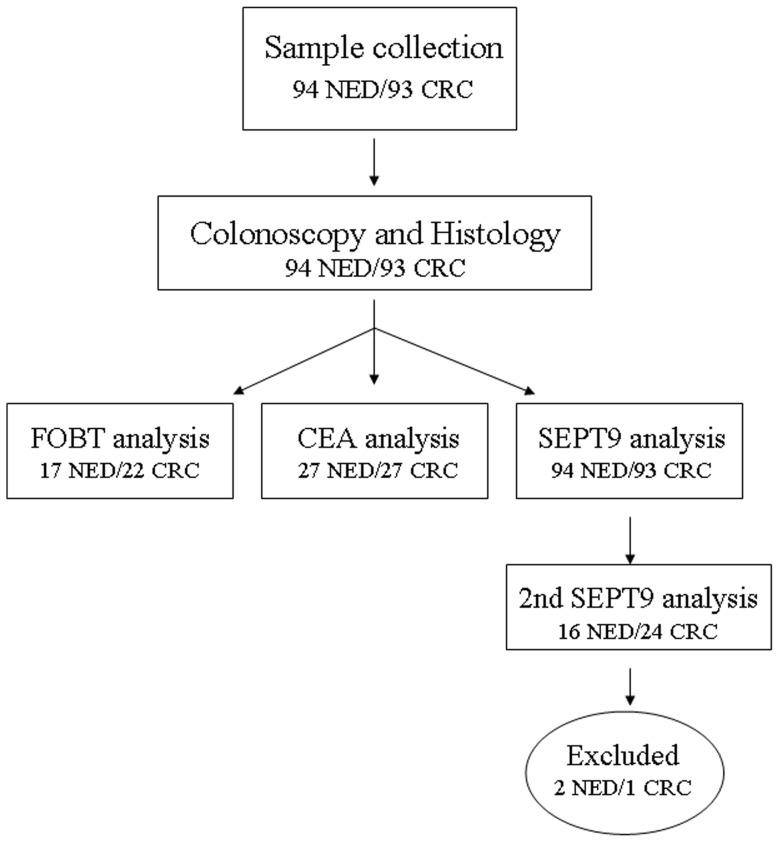
Figure 1. Study design and sample number for each step of the assay.
94 NED and 93 CRC plasma samples were collected. Forty patient samples (16 NED and 24 CRC) were measured twice for SEPT9 validation purposes. 2 NED and 1 CRC samples yielded both Septin 9 positive and negative results; hence they were excluded from the study. Furthermore samples for FOBT and CEA were collected retrospectively. CRC = colorectal cancer; NED = no evidence of disease (healthy control); SEPT9 = Septin 9.