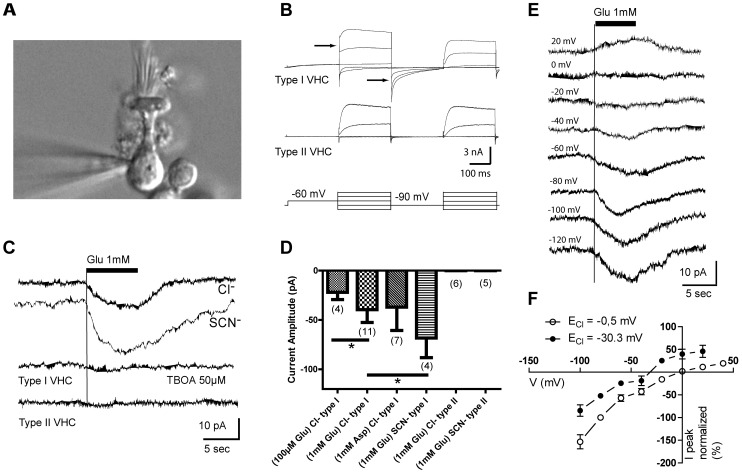
Figure 1. Transporter-mediated chloride conductance in partially isolated type I vestibular hair cells (VHCs).
(A) Differential interference contrast micrograph of a recording electrode tip approaching the base of an amphora-shaped, type I utricular hair cell. (B) Electrophysiological protocol used to identify type I hair cells (upper traces) versus type II hair cells (bottom traces). As previously described [27], only type I cells exhibit IKL, an outwardly rectifying current activated at rest that was evidenced by an instantaneous current upon stepping to higher voltages (leftmost arrow) and deactivated by hyperpolarization (rightmost arrow). (C) Inward current evoked in different type I VHCs voltage-clamped at −80 mV upon application of glutamate (uppermost trace); inward current enhanced by substitution of thiocyanate (SCN–) for chloride (second trace); the glutamate-evoked current was blocked following application of dl-TBOA (third trace). Application of glutamate did not evoke a current in type II VHCs (bottom trace). (D) Glutamate activated current under different conditions (glutamate or aspartate at various concentrations, chloride or thiocyanate anion intracellularly, and type I or type II VHCs). Each bar represents mean ± sd. Brackets with asterisk indicate Mann-Whitney U comparisons (p<0.05 for glutamate, 100 µM vs 1 mM and 1 mM Cl- vs 1 mM SCN-); sample sizes (n) in parentheses. (E) Currents evoked by 1 mM glutamate application at various holding potentials on a type I VHC, from −120 to +20 mV in 20 mV steps. (F) Current-voltage relationship of glutamate-evoked responses obtained with E Cl = −0.5 mV (n = 7) and E Cl = −30.3 mV (n = 5). Peak currents at each holding potential were normalized to responses at −80 mV. Dots represent mean ± sem. When no bar is shown, the SEM was too small.