Figure 6. Putative mechanisms of the ZEA biodegradation by Rhodococcus pyridinivorans K408.
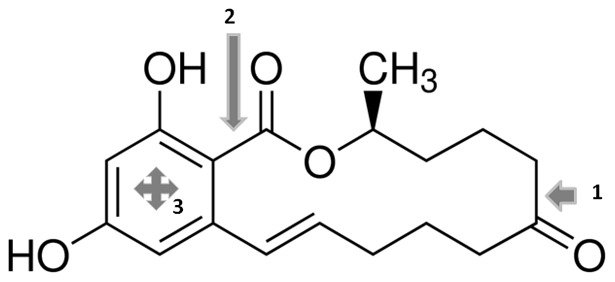
The arising metabolites and the zearalenone degradation mechanism of Rhodococcus pyridinivorans K408 are unknown yet. However, previous studies suggest various ways how zearalenone could be degraded by microbes. Trichosporon mycotoxinivorans (1) and Clonostachys rosea IFO 7063 (2) are able to open the lactone ring resulting non-estrogenic metabolites which are 1-(3,5-dihydroxyphenyl)-10′-hydroxy-1-undecen-6′-one and (5S)-5-({2,4-dihydroxy-6-[(1E)-5-hydroxypent-1-en-1-yl]benzoyl}oxy) hexanoic acid, respectively. In addition, Rhodococcus pyridinivorans K408 have versatile metabolic pathways and the most of them are able to degrade aromatic compounds. It suggests that opening the aromatic ring is also a possible way to degrade zearalenone (3).
